Page 5 - Application Notebook - Solution for Food Development
P. 5
Application No.L459
News
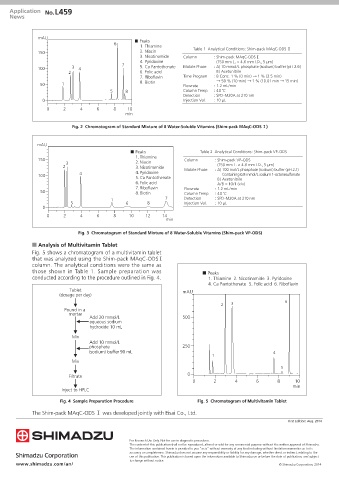
mAU ■ Peaks
6
1. Thiamine Table 1 Analytical Conditions: Shim-pack MAqC-ODS Ⅰ
150 2. Niacin
3. Nicotinamide Column : Shim-pack MAqC-ODS Ⅰ
4. Pyridoxine (150 mm L. × 4.6 mm I.D., 5 µm)
7 5. Ca Pantothenate Mobile Phase : A) 10 mmol/L phosphate (sodium) buffer (pH 2.6)
100 3 4
2 6. Folic acid B) Acetonitrile
7. Riboflavin Time Program : B Conc. 1 % (0 min) → 1 % (2.5 min)
→ 50 % (10 min) → 1 % (10.01 min → 15 min)
50 1 8. Biotin Flowrate : 1.2 mL/min
5 8 Column Temp. : 40 ˚C
Detection : SPD-M20A at 210 nm
0 Injection Vol. : 10 µL
0 2 4 6 8 10
min
Fig. 2 Chromatogram of Standard Mixture of 8 Water-Soluble Vitamins (Shim-pack MAqC-ODS Ⅰ)
mAU
■ Peaks Table 2 Analytical Conditions: Shim-pack VP-ODS
1. Thiamine
150 Column : Shim-pack VP-ODS
3 2. Niacin (150 mm L. × 4.6 mm I.D., 5 µm)
2 3. Nicotinamide Mobile Phase : A) 100 mol/L phosphate (sodium) buffer (pH 2.1)
4 4. Pyridoxine Containing 0.8 mmol/L sodium 1-octanesulfonate
100
5. Ca Pantothenate B) Acetonitrile
6. Folic acid A/B = 10/1 (v/v)
7. Riboflavin Flowrate : 1.2 mL/min
50 8. Biotin Column Temp. : 40 ˚C
1 7 Detection : SPD-M20A at 210 nm
5 6 8 Injection Vol. : 10 µL
0
0 2 4 6 8 10 12 14
min
Fig. 3 Chromatogram of Standard Mixture of 8 Water-Soluble Vitamins (Shim-pack VP-ODS)
n Analysis of Multivitamin Tablet
Fig. 5 shows a chromatogram of a multivitamin tablet
that was analyzed using the Shim-pack MAqC-ODS Ⅰ
column. The analytical conditions were the same as
those shown in Table 1. Sample preparation was ■ Peaks
conducted according to the procedure outlined in Fig. 4. 1. Thiamine 2. Nicotinamide 3. Pyridoxine
4. Ca Pantothenate 5. Folic acid 6. Riboflavin
Tablet mAU
(dosage per day)
2 3 6
Pound in a
mortar
Add 20 mmol/L 500
aqueous sodium
hydroxide 10 mL
Mix
Add 10 mmol/L
phosphate 250
(sodium) buffer 90 mL 4
1
Mix
5
Filtrate 0
0 2 4 6 8 10
min
Inject to HPLC
Fig. 4 Sample Preparation Procedure Fig. 5 Chromatogram of Multivitamin Tablet
The Shim-pack MAqC-ODS Ⅰ was developed jointly with Eisai Co., Ltd.
First Edition: Aug. 2014
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2014