Page 34 - Application Handbook - TOC
P. 34
Sum parameter – Total Organic Carbon
TOC – Determination in brines
SCA-130-304
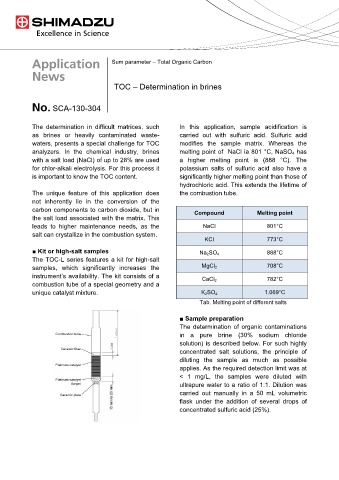
The determination in difficult matrices, such In this application, sample acidification is
as brines or heavily contaminated waste- carried out with sulfuric acid. Sulfuric acid
waters, presents a special challenge for TOC modifies the sample matrix. Whereas the
analyzers. In the chemical industry, brines melting point of NaCl ia 801 °C, NaSO 4 has
with a salt load (NaCl) of up to 28% are used a higher melting point is (888 °C). The
for chlor-alkali electrolysis. For this process it potassium salts of sulfuric acid also have a
is important to know the TOC content. significantly higher melting point than those of
hydrochloric acid. This extends the lifetime of
The unique feature of this application does the combustion tube.
not inherently lie in the conversion of the
carbon components to carbon dioxide, but in Compound Melting point
the salt load associated with the matrix. This
leads to higher maintenance needs, as the NaCl 801°C
salt can crystallize in the combustion system.
KCl 773°C
■ Kit or high-salt samples Na 2SO 4 888°C
The TOC-L series features a kit for high-salt
samples, which significantly increases the MgCl 2 708°C
instrument’s availability. The kit consists of a
CaCl 2 782°C
combustion tube of a special geometry and a
unique catalyst mixture. K 2SO 4 1.069°C
Tab. Melting point of different salts
■ Sample preparation
The determination of organic contaminations
in a pure brine (30% sodium chloride
solution) is described below. For such highly
concentrated salt solutions, the principle of
diluting the sample as much as possible
applies. As the required detection limit was at
< 1 mg/L, the samples were diluted with
ultrapure water to a ratio of 1:1. Dilution was
carried out manually in a 50 mL volumetric
flask under the addition of several drops of
concentrated sulfuric acid (25%).