Page 73 - Application Handbook - Liquid Chromatography
P. 73
Application No.L500
News
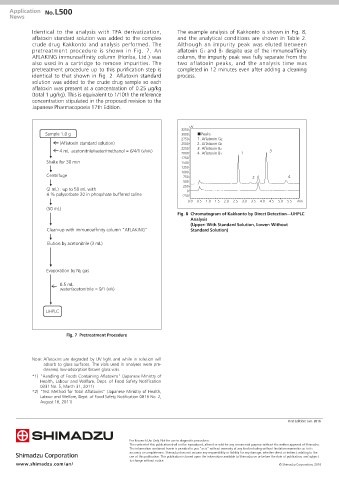
Identical to the analysis with TFA derivatization, The example analysis of Kakkonto is shown in Fig. 8,
aflatoxin standard solution was added to the complex and the analytical conditions are shown in Table 2.
crude drug Kakkonto and analysis performed. The Although an impurity peak was eluted between
pretreatment procedure is shown in Fig. 7. An aflatoxin G1 and B2 despite use of the immunoaffinity
AFLAKING immunoaffinity column (Horiba, Ltd.) was column, the impurity peak was fully separate from the
also used in a cartridge to remove impurities. The two aflatoxin peaks, and the analysis time was
pretreatment procedure up to this purification step is completed in 12 minutes even after adding a cleaning
identical to that shown in Fig. 2. Aflatoxin standard process.
solution was added to the crude drug sample so each
aflatoxin was present at a concentration of 0.25 µg/kg
(total 1 µg/kg). This is equivalent to 1/10th the reference
concentration stipulated in the proposed revision to the
Japanese Pharmacopoeia 17th Edition.
uV
3250
Sample 1.0 g 3000 ■Peaks
2750 1. Aflatoxin G2
(Aflatoxin standard solution) 2500 2. Aflatoxin G1
2250 3. Aflatoxin B2
4 mL acetonitrile/water/methanol = 6/4/1 (v/v/v) 3
2000 4. Aflatoxin B1 1
1750
Shake for 30 min 1500
1250
1000
Centrifuge 750 2 4
500
250
(2 mL) : up to 50 mL with 0
4 % polysorbate 20 in phosphate buffered saline -250
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 min
(50 mL)
Fig. 8 Chromatogram of Kakkonto by Direct Detection—UHPLC
Analysis
(Upper: With Standard Solution, Lower: Without
Clean-up with immunoaffinity column “AFLAKING” Standard Solution)
Elution by acetonitrile (3 mL)
Evaporation by N2 gas
0.5 mL
water/acetonitrile = 9/1 (v/v)
UHPLC
Fig. 7 Pretreatment Procedure
Note: Aflatoxins are degraded by UV light and while in solution will
adsorb to glass surfaces. The vials used in analyses were pre-
cleaned, low-adsorption brown glass vials.
*1) "Handling of Foods Containing Aflatoxins" (Japanese Ministry of
Health, Labour and Welfare, Dept. of Food Safety Notification
0331 No. 5, March 31, 2011)
*2) "Test Method for Total Aflatoxins" (Japanese Ministry of Health,
Labour and Welfare, Dept. of Food Safety Notification 0816 No. 2,
August 16, 2011)
First Edition: Jan. 2016
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2016