Page 77 - Application Handbook - Liquid Chromatography
P. 77
Application No.L481
News
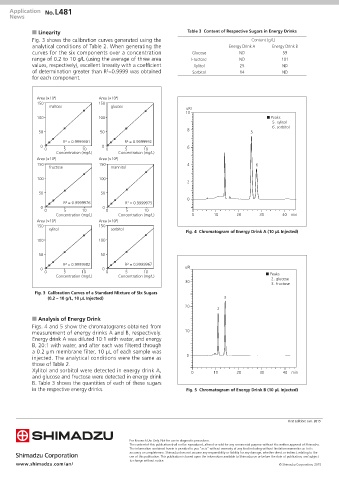
■ Linearity Table 3 Content of Respective Sugars in Energy Drinks
Fig. 3 shows the calibration curves generated using the Content (g/L)
analytical conditions of Table 2. When generating the Energy Drink A Energy Drink B
curves for the six components over a concentration Glucose ND 59
range of 0.2 to 10 g/L (using the average of three area Fructose ND 101
values, respectively), excellent linearity with a coefficient Xylitol 25 ND
2
of determination greater than R =0.9999 was obtained Sorbitol 14 ND
for each component.
Area (×10 ) 4 Area (×10 ) 4
150 150
m e s o t l a e s oc u l g uRI
10
100 100 ■ Peaks
5. xylitol
6. sorbitol
50 50 8 5
R² = 0.9999981 R² = 0.9999992
0 0 6
0 5 10 0 5 10
Concentration (mg/L) Concentration (mg/L)
Area (×10 ) 4 Area (×10 ) 4
150 150 4 6
fructose mannitol
100 100
2
50 50
0
R² = 0.9999976 R² = 0.9999975
0 0
0 5 10 0 5 10
Concentration (mg/L) Concentration (mg/L) 0 10 20 30 40 min
Area (×10 ) 4 Area (×10 ) 4
150 150
xylitol sorbitol
Fig. 4 Chromatogram of Energy Drink A (10 µL Injected)
100 100
50 50
R² = 0.9999982 R² = 0.9999967
0 0 uRI
0 5 10 0 5 10 ■ Peaks
Concentration (mg/L) Concentration (mg/L) 2. glucose
30
3. fructose
Fig. 3 Calibration Curves of a Standard Mixture of Six Sugars
(0.2 – 10 g/L, 10 µL Injected) 3
20
2
■ Analysis of Energy Drink
Figs. 4 and 5 show the chromatograms obtained from
measurement of energy drinks A and B, respectively. 10
Energy drink A was diluted 10:1 with water, and energy
B, 20:1 with water, and after each was filtered through
a 0.2 µm membrane filter, 10 µL of each sample was
injected. The analytical conditions were the same as 0
those of Table 2.
Xylitol and sorbitol were detected in energy drink A, 0 10 20 30 40 min
and glucose and fructose were detected in energy drink
B. Table 3 shows the quantities of each of these sugars
in the respective energy drinks. Fig. 5 Chromatogram of Energy Drink B (10 µL Injected)
First Edition: Jan. 2015
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2015