Page 9 - Clinical-Medical Solution for Clinical Chemistry
P. 9
Application No. C153
News
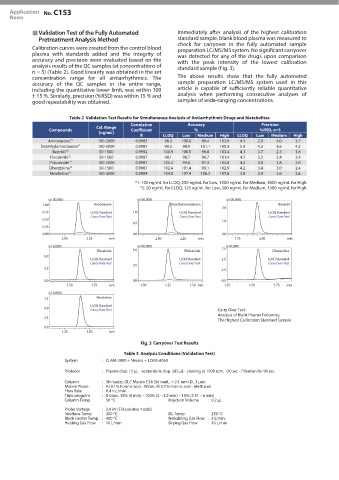
Validation Test of the Fully Automated Immediately after analysis of the highest calibration
Pretreatment Analysis Method standard sample, blank blood plasma was measured to
check for carryover in the fully automated sample
Calibration curves were created from the control blood preparation LC/MS/MS system. No significant carryover
plasma with standards added and the integrity of was detected for any of the drugs upon comparison
accuracy and precision were evaluated based on the with the peak intensity of the lowest calibration
analysis results of the QC samples (at concentrations of standard sample (Fig. 3).
n = 5) (Table 2). Good linearity was obtained in the set
concentration range for all antiarrhythmics. The The above results show that the fully automated
accuracy of the QC samples in the entire range, sample preparation LC/MS/MS system used in this
including the quantitative lower limit, was within 100 article is capable of sufficiently reliable quantitative
± 15 %. Similarly, precision (%RSD) was within 15 % and analysis when performing consecutive analyses of
good repeatability was obtained. samples of wide-ranging concentrations.
Table 2 Validation Test Results for Simultaneous Analysis of Antiarrhythmic Drugs and Metabolites
Correlation Accuracy Precision
Cal. Range
Compounds Coefficient % %RSD, n=5
[ng/mL]
R LLOQ Low Medium High LLOQ Low Medium High
Amiodarone 100-3000 0.9983 98.3 100.6 99.4 103.9 4.1 2.9 3.0 2.7
*1
*1
Desethylamiodarone 100-3000 0.9987 99.2 98.9 101.1 100.3 5.3 4.2 3.6 4.2
Bepridil 50-1500 0.9992 100.9 100.5 96.6 103.4 4.1 3.7 2.3 1.8
*2
*2
Flecainide 50-1500 0.9987 98.1 98.7 96.7 101.4 4.7 3.3 2.4 2.4
Pilsicainide 100-3000 0.9987 100.4 99.6 97.3 104.8 4.0 3.0 1.8 2.0
*1
*2
Cibenzoline 50-1500 0.9987 102.4 101.4 99.1 102.9 4.2 3.4 3.0 2.4
Mexiletine 100-3000 0.9984 104.5 107.4 106.3 107.8 3.8 3.9 2.6 2.6
*1
*1: 100 ng/mL for LLOQ, 250 ng/mL for Low, 1000 ng/mL for Medium, 3000 ng/mL for High
*2: 50 ng/mL for LLOQ, 125 ng/mL for Low, 500 ng/mL for Medium, 1500 ng/mL for High
Carry Over Test:
Analysis of Blank Plasma Following
The Highest Calibration Standard Sample
Carryover Test Results
Table 3 Analysis Conditions (Validation Test)
System : CLAM-2000 + Nexera + LCMS-8060
Protocol : Plasma disp. 15 μL - acetonitrile disp. 285 μL - shaking at 1900 rpm, 120 sec - filtration for 90 sec
Column : Shimadzu GLC Mastro C18 (50 mmL. × 2.1 mmI.D., 3 μm)
Mobile Phase : A) 0.1% Formic acid - Water, B) 0.1% Formic acid - Methanol
Flow Rate : 0.4 mL/min
Time program : B Conc. 10% (0 min) – 100% (2 – 3.5 min) – 10% (3.51 – 6 min)
Column Temp. 50 °C Injection Volume : 0.2 μL
Probe Voltage : 2.0 kV (ESI-positive mode)
Interface Temp. : 300 °C DL Temp. : 250 °C
Block Heater Temp. : 400 °C Nebulizing Gas Flow : 3 L/min
Heating Gas Flow : 10 L/min Drying Gas Flow : 10 L/min