Page 20 - Shimadzu’s Solutions for Impurities Analysis
P. 20
Support for Analytical Instrument
Support from Shimadzu
Qualification
Validation Support
˙ Trends in Analytical Instrument Qualification
The U.S. Pharmacopoeia includes Analytical Instrument procedure from Design Qualification (DQ) at the time of
Qualification (AIQ) in the addendum USP31. AIQ indicates planning, Performance Qualification (PQ) at the start of
qualification and maintenance throughout the life cycle of actual application, and maintaining instrument performance
analytical instruments including the instrument qualification after the start of operation.
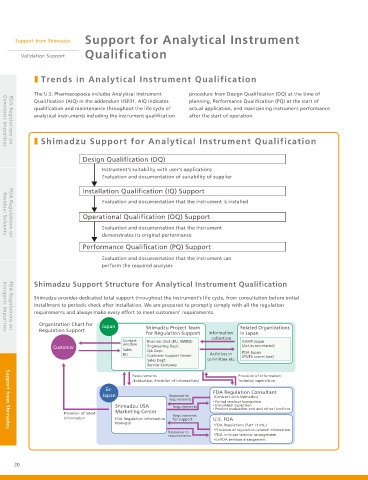
˙ Shimadzu Support for Analytical Instrument Qualification
FDA Regulations on
Genotoxic Impurities
Design Qualification (DQ)
Instrument's suitability with user's applications
Evaluation and documentation of suitability of supplier
Installation Qualification (IQ) Support
Evaluation and documentation that the instrument is installed
Operational Qualification (OQ) Support
Evaluation and documentation that the instrument
demonstrates its original performance
Residual Solvents
FDA Regulations on
Performance Qualification (PQ) Support
Evaluation and documentation that the instrument can
perform the required analyses
Shimadzu Support Structure for Analytical Instrument Qualification
Shimadzu provides dedicated total support throughout the instrument's life cycle, from consultation before initial
installment to periodic check after installation. We are prepared to promptly comply with all the regulation
requirements and always make every effort to meet customers’ requirements.
Organization Chart for Japan
Regulation Support Shimadzu Project Team Related Organizations
FDA Regulations on
Inorganic Impurities
for Regulation Support Information in Japan
collection
Contact Business Unit (BU, NWBD) GAMP Japan
window (Act as secretariat)
Customer Engineering Dept.
Sales QA Dept. PDA Japan
BU Customer Support Center Activities in (ER/ES committee)
Sales Dept. committee etc.
Service Company
Requirements Provision of information,
(Evaluation, Provision of information) Technical supervision
Ex-
FDA Regulation Consultant
Japan Response to (Contract with Shimadzu)
requirements
ɾ Period seminar instruction
Shimadzu USA Requirements ɾ Simulated inspection
Product evaluation and and other functions
Marketing Center ɾ
Provision of latest
Requirements
information FDA Regulation Information for support U.S. FDA
Manager
ɾ
FDA Regulations (Part 11 etc.)
ɾ
Provision of regulation-related information
Support from Shimadzu
Response to
FDA in-house seminar arrangement
requirements ɾ
ɾ
Ex-FDA seminar arrangement
20