Page 19 - Shimadzu’s Solutions for Impurities Analysis
P. 19
FDA Regulations on
Inorganic Impurities
Atomic Absorption Spectrophotometer
Data
Analysis of Lead in White Sugar Using the AA-7000 and GFA-7000
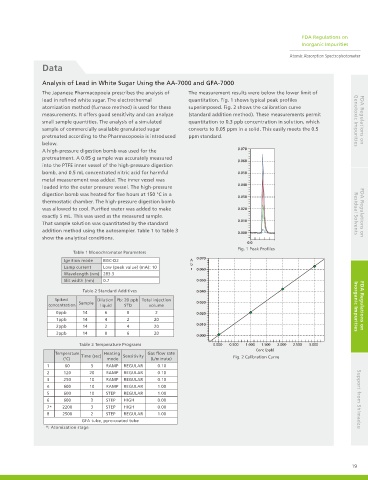
The Japanese Pharmacopoeia prescribes the analysis of The measurement results were below the lower limit of
lead in refined white sugar. The electrothermal quantitation. Fig. 1 shows typical peak profiles
atomization method (furnace method) is used for these superimposed. Fig. 2 shows the calibration curve
measurements. It offers good sensitivity and can analyze (standard addition method). These measurements permit
small sample quantities. The analysis of a simulated quantitation to 0.3 ppb concentration in solution, which Genotoxic Impurities FDA Regulations on
sample of commercially available granulated sugar converts to 0.05 ppm in a solid. This easily meets the 0.5
pretreated according to the Pharmacopoeia is introduced ppm standard.
below.
A high-pressure digestion bomb was used for the 0.070
pretreatment. A 0.05 g sample was accurately measured
0.060
into the PTFE inner vessel of the high-pressure digestion
bomb, and 0.5 mL concentrated nitric acid for harmful 0.050
metal measurement was added. The inner vessel was
0.040
loaded into the outer pressure vessel. The high-pressure
digestion bomb was heated for five hours at 150 °C in a
0.030
thermostatic chamber. The high-pressure digestion bomb
was allowed to cool. Purified water was added to make 0.020
exactly 5 mL. This was used as the measured sample. Residual Solvents FDA Regulations on
0.010
That sample solution was quantitated by the standard
addition method using the autosampler. Table 1 to Table 3
0.000
show the analytical conditions.
0.0
Fig. 1 Peak Profiles
Table 1 Monochromator Parameters
Ignition mode BGC-D2 A 0.070
b
Lamp current Low (peak value) (mA): 10 s 0.060
Wavelength (nm) 283.3
Slit width (nm) 0.7 0.050
Table 2 Standard Additives 0.040
Spiked Dilution Pb: 20 ppb Total injection
Sample 0.030
concentration liquid STD volume Inorganic Impurities FDA Regulations on
0ppb 14 6 0 2 0.020
1ppb 14 4 2 20
0.010
2ppb 14 2 4 20
3ppb 14 0 6 20
0.000
Table 3 Temperature Programs 0.000 0.500 1.000 1.500 2.000 2.500 3.000
Conc (ppb)
Temperature Time (sec) Heating Sensitivity Gas flow rate
(°C) mode (L/minute) Fig. 2 Calibration Curve
1 60 3 RAMP REGULAR 0.10
2 120 20 RAMP REGULAR 0.10
3 250 10 RAMP REGULAR 0.10
4 600 10 RAMP REGULAR 1.00
5 600 10 STEP REGULAR 1.00
6 600 3 STEP HIGH 0.00 Support from Shimadzu
7* 2200 3 STEP HIGH 0.00
8 2500 2 STEP REGULAR 1.00
GFA tube, pyro-coated tube
*: Atomization stage
19