Page 8 - Food&Beverages-Food Development
P. 8
Amino Acids Organic Acids
Of all the amino acids, glutamic acid is widely known as a D-amino acids have different flavors, with D-amino acids Organic acids contained in food products are important development. However, know-how is needed to detect various
component of the umami taste (delicious taste). Further, the exerting a broad influence on the flavor of fermented foods components that contribute to their deliciousness, including organic acids specifically and with high sensitivity in samples
types and component ratios of amino acids largely control the and aged foods. Numerous amino acids are being analyzed for their sourness and umami tastes. In addition, recently, they have with many impurities such as food products. The organic acid
flavor of this food product. For example, glycine and alanine are purposes of food development and quality control, with new become a topic of interest for their digestive stimulant and analysis system is used in the field of food development as a
sweet, valine and leucine are bitter, and aspartic acid and components of the umami taste and sweetening agents antibacterial effects. simultaneous analysis method for various organic acids.
glutamic acid are sour. expected. The analysis of organic acids is a useful approach to food
In addition, in recent years, it has been reported that L- and
High-Performance Liquid Chromatograph
Organic Acid Analysis System Food Texture
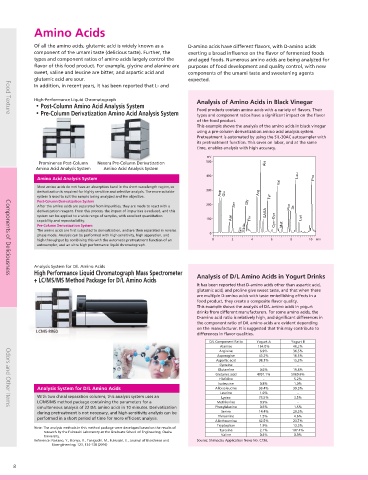
High-Performance Liquid Chromatograph Analysis of Amino Acids in Black Vinegar Analysis of Organic Acids in Food Products
・Post-Column Amino Acid Analysis System
・Pre-Column Derivatization Amino Acid Analysis System Food products contain amino acids with a variety of flavors. Their Organic acids such as citric acid and acetic acid shape the flavor and
types and component ratios have a significant impact on the flavor
Food Texture
of the food product. aroma of food products in various ways.
This example shows the analysis of organic acids in food product
This example shows the analysis of the amino acids in black vinegar samples. It was confirmed that organic acids can be analyzed with
using a pre-column derivatization amino acid analysis system. high accuracy, and with no interference from impurities.
Pretreatment is automated by using the SIL-30AC autosampler with
its pretreatment function. This saves on labor, and at the same
time, enables analysis with high accuracy.
mV
4.0
mV 3.5 2 7
Prominence Post-Column Nexera Pre-Column Derivatization 500 3.0
Amino Acid Analysis System Amino Acid Analysis System 2.5 3
2.0
400 1.5 1
Amino Acid Analysis System 1.0 4 5
Organic Acid Analysis System 0.5 6
Most amino acids do not have an absorption band in the short wavelength region, so 0
derivatization is required for highly sensitive and selective analysis. The more suitable 300 This organic acid analysis system features excellent selectivity and 0.0 1.0 2.0 3.0 4.0 5.0 min
system is used to suit the sample being analyzed and the objective. sensitivity due to the post-column pH-buffered electric conductivity Tomato Extract White Wine (UHPLC analysis)
Post-Column Derivatization System detection method.
After the amino acids are separated from impurities, they are made to react with a 200 The organic acids are separated by ion exclusion chromatography. Then 1. Citric acid 4. Acetic acid 1. Formic acid 5. Citric acid
2. Succinic acid 5. Carbonic acid
2. Malic acid
6. Pyroglutamic acid
derivatization reagent. From this process, the impact of impurities is reduced, and this 3. Lactic acid 3. Lactic acid 7. Succinic acid
system can be applied to a wide range of samples, with excellent quantitation a pH buffering reagent is continuously added to the column eluate. 4. Acetic acid
capability and reproducibility. 100 Since the pH is maintained at nearly neutral, the organic acids are
Pre-Column Derivatization System dissociated and detected via electric conductivity. This is suitable for the
The amino acids are first subjected to derivatization, and are then separated in reverse analysis of samples with many impurities. Source: Shimadzu Application News No. L208 and No. L436.
phase mode. Analysis can be performed with high sensitivity, high separation, and 0
high throughput by combining this with the automatic pretreatment function of an 0 2 4 6 8 10 min Components of Deliciousness
autosampler, and an ultra high performance liquid chromatograph.
Analysis System for D/L Amino Acids Sugar
High Performance Liquid Chromatograph Mass Spectrometer Analysis of D/L Amino Acids in Yogurt Drinks
Components of Deliciousness
+ LC/MS/MS Method Package for D/L Amino Acids As the basis for sweetness, sugar is one of the most familiar sweetening agents and functional foods are a focus of
It has been reported that D-amino acids other than aspartic acid,
glutamic acid, and proline give sweet taste, and that when there components used as raw ingredients and flavoring agents in attention, and research and development of sugar are actively
are multiple D-amino acids with taste embellishing effects in a food products. However, although they are all referred to as conducted. The reducing sugar analysis system is used in the
food product, they create a composite flavor quality. sugar, there are many varieties, and they differ significantly in field of food development as a high-sensitivity analysis method
This example shows the analysis of D/L amino acids in yogurt their sweetness and bioactivity. In addition, diets and health for various reducing sugars from brewed food products and
drinks from different manufacturers. For some amino acids, the consciousness are in favor these days, the development of other samples.
D-amino acid ratio is relatively high, and significant differences in
the component ratio of D/L amino acids are evident depending
on the manufacturer. It is suggested that this may contribute to High-Performance Liquid Chromatograph
LCMS-8060 Reducing Sugar Analysis System
differences in flavor qualities.
Analysis of Sugars in Food Products
D/L Component Ratio Yogurt A Yogurt B
Alanine 164.0% 40.2% This example shows the analysis of sugars in foods using the
Arginine 6.9% 36.5% reducing sugar analysis system.
Asparagine 43.2% 16.5% It was confirmed that high-sensitivity analysis can be performed
Aspartic acid 38.1% 15.3%
Cysteine - - with high selectivity for trace sugar content, in food product
Glutamine 0.6% 19.8% samples with many impurities.
Glutamic acid 4091.1% 5069.6%
Histidine - 5.2% uRI Peaks mV Odors and Other Items
Isoleucine 0.8% 1.0% 30 2. Sucrose 13 12 Peaks 6 7
1.Rhamnose
3. Glucose
Analysis System for D/L Amino Acids Alloisoleucine 59.4% 39.3% 2 4. Fructose 11 10 2.Ribose
3.Fucose
4.Xylose
Leucine 1.0% - 9 5.Arabinose
6.Fructose
With two chiral separation columns, this analysis system uses an Lysine 73.5% 3.5% 20 3 4 8 7.Glucose
7
LC/MS/MS method package containing the parameters for a Methionine 0.9% - 6
simultaneous analysis of 22 D/L amino acids in 10 minutes. Derivatization Phenylalanine 0.5% 1.5% 10 5 4 5
Odors and Other Items
during pretreatment is not necessary, and high-sensitivity analysis can be Serine 14.4% 29.3% Reducing Sugar Analysis System 3 2 1 3 4
performed in a short period of time for more efficient analysis. Threonine 1.5% 4.6% This reducing sugar analysis system uses the post-column fluorescence 0 1 2
Allothreonine 42.5% 23.7% 0
Tryptophan 1.9% 13.3% derivatization detection method with arginine as the reaction reagent. 0.0 5.0 10.0 15.0 20.0 25.0 min 0.0 5.0 10.0 15.0 20.0 25.0 min
Note: The analysis methods in this method package were developed based on the results of
research by the Fukusaki Laboratory at the Graduate School of Engineering, Osaka Tyrosine 2.1% 107.4% It is capable of high-sensitivity analysis with high selectivity for reducing Orange Juice Vinegar
University. Valine 0.4% 0.9% sugar, even in samples with very little sugar content and many
Reference: Nakano, Y., Konya, Y., Taniguchi, M., Fukusaki, E., Journal of Bioscience and Source: Shimadzu Application News No. C156. impurities. Source: Shimadzu Application News No. L467 and No. L382.
Bioengineering, 123, 134-138 (2016)
8 9