Page 23 - Shimadzu Journal vol.8 Issue2
P. 23
Hydrocarbon Processing Industry
Figure 3. Spectra of sulfur Kα showing the effect of the use of X-ray filters to minimize detection of scattered X-rays.
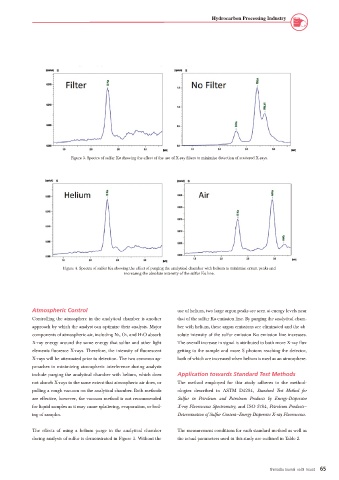
Figure 4. Spectra of sulfur Kα showing the effect of purging the analytical chamber with helium to minimize errant peaks and
increasing the absolute intensity of the sulfur Kα line.
Atmospheric Control use of helium, two large argon peaks are seen at energy levels near
Controlling the atmosphere in the analytical chamber is another that of the sulfur Kα emission line. By purging the analytical cham-
approach by which the analyst can optimize their analysis. Major ber with helium, these argon emissions are eliminated and the ab-
components of atmospheric air, including N2, O2, and H2O absorb solute intensity of the sulfur emission Kα emission line increases.
X-ray energy around the same energy that sulfur and other light The overall increase in signal is attributed to both more X-ray flux
elements fluoresce X-rays. Therefore, the intensity of fluorescent getting to the sample and more S photons reaching the detector,
X-rays will be attenuated prior to detection. The two common ap- both of which are increased when helium is used as an atmosphere.
proaches to minimizing atmospheric interference during analysis
include purging the analytical chamber with helium, which does Application towards Standard Test Methods
not absorb X-rays to the same extent that atmospheric air does, or The method employed for this study adheres to the method-
pulling a rough vacuum on the analytical chamber. Both methods ologies described in ASTM D4294, Standard Test Method for
are effective, however, the vacuum method is not recommended Sulfur in Petroleum and Petroleum Products by Energy-Dispersive
for liquid samples as it may cause splattering, evaporation, or boil- X-ray Fluorescence Spectrometry, and ISO 5784, Petroleum Products—
ing of samples. Determination of Sulfur Content—Energy Dispersive X-ray Fluorescence.
The effects of using a helium purge in the analytical chamber The measurement conditions for each standard method as well as
during analysis of sulfur is demonstrated in Figure 3. Without the the actual parameters used in this study are outlined in Table 2.
65
Shimadzu Journal vol.8 Issue2 65