Page 6 - Shimadzu Journal vol.7 Issue2
P. 6
Food Development
Determination of mineral oil residues in food
Removing natural occurring alkanes
Erich Leitner, Andrea Walzl, Graz University of Technology, Institute of Analytical Chemistry and Food Chemistry, Research group “Food Chemistry and Human Sensory
Analysis“, 8010 Graz, Austria
Uwe Oppermann, Shimadzu Europa GmbH, Marketing Europe, Analytical BU, Food Market, 47269 Duisburg, Germany
Introduction
Mineral oil (MO) residues in food raised public concern due to concentration range from 15 - 35 %. The determination of MOSH
some elevated concentrations up to several thousand milligrams and MOAH in food can be done by an automated LC-GC-FID system
[1]
per kilogram food . Due to the chemical structures two groups of (liquid chromatography–gas chromatography–flame ionization detection)
MOs can be differentiated. Mineral oil saturated hydrocarbons for routine analysis. Unfortunately, some food material like rice or
(MOSH) consist of linear and branched alkanes, and alkyl-substituted chocolate contain natural occurring (odd numbered) alkanes in the
cyclo-alkanes, whilst mineral oil aromatic hydrocarbons (MOAH) range of C23 - C33 which can interfere and heavily disturb the analysis
include mainly alkyl-substituted polyaromatic hydrocarbons. of the MOSH fraction. These interferences can be removed by flash
[2]
Technical grades of mineral contain aromatic hydrocarbons in a chromatography on aluminium oxide columns .
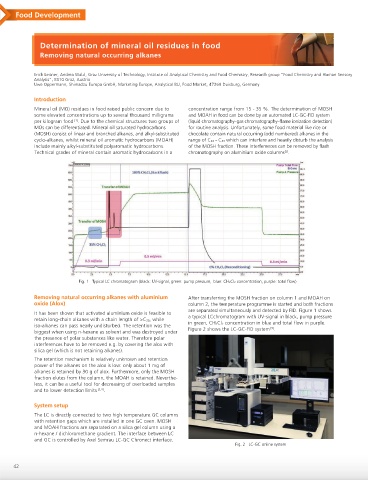
Fig. 1 Typical LC chromatogram (black: UV-signal, green: pump pressure, blue: CH2Cl2 concentration, purple: total flow)
Removing natural occurring alkanes with aluminium After transferring the MOSH fraction on column 1 and MOAH on
oxide (Alox) column 2, the temperature programme is started and both fractions
are separated simultaneously and detected by FID. Figure 1 shows
It has been shown that activated aluminium oxide is feasible to a typical LCchromatogram with UV-signal in black, pump pressure
retain long-chain alkanes with a chain length of >C20, while in green, CH2Cl2 concentration in blue and total flow in purple.
iso-alkanes can pass nearly undisturbed. The retention was the [5]
biggest when using n-hexane as solvent and was destroyed under Figure 2 shows the LC-GC-FID system .
the presence of polar substances like water. Therefore polar
interferences have to be removed e.g. by covering the alox with
silica gel (which is not retaining alkanes).
The retention mechanism is relatively unknown and retention
power of the alkanes on the alox is low: only about 1 mg of
alkanes is retained by 30 g of alox. Furthermore, only the MOSH
fraction elutes from the column, the MOAH is retained. Neverthe-
less, it can be a useful tool for decreasing of overloaded samples
and to lower detection limits [3,4] .
System setup
The LC is directly connected to two high temperature GC columns
with retention gaps which are installed in one GC oven. MOSH
and MOAH fractions are separated on a silica gel column using a
n-hexane / dichloromethane gradient. The interface between LC
and GC is controlled by Axel Semrau LC-GC Chronect interface.
Fig. 2 LC-GC online system
42