Page 5 - MUP-3100
P. 5
Stable performance eliminates differences Provides Labor Savings and Reduces Downtime
between analysts, providing peace of mind
Simply Place the Sample and Reagents in the Instrument to Automatically
Implement Pretreatment
Simply place the prepared reagent
and the culture supernatants or
other samples into the prescribed
place for the instrument and select
pretreatment to implement the
Precisely Automated Protocols Eliminate Human Error process automatically, quickly and
accurately. The MUP-3100 allows for
setting a maximum of 24 samples at
Pretreatment is required for LC analysis of profiles for antibody-derived glycans, including purification of the antibodies, glycan one time and enables processing
cut-out, and fluorescent labeling. The MUP-3100 Fully Automated Sample Preparation Module for Glycan Analysis is equipped twice a day. The MUP-3100 not only
with functions to perform dispensing, the addition of reagents, heating, centrifugation, solid phase column extraction, and lessens the burden on the analyst,
sample delivery, thereby automating the entire pretreatment sequence. but also enables pretreatment at
Pretreatment of antibody-derived glycans in culture supernatants involves repeated operations including dispensing, the night or on holidays, thereby
addition of reagents, centrifugation, and heating. With solid phase column extraction using an antibody trap carrier, rinsing accelerating the workflow.
must be performed multiple times, which is labor intensive.
The MUP-3100 reliably implements pretreatment using the Auto-EZGlyco mAb-N Kit for SHIMADZU protocol. This eliminates
human errors, such as forgetting to add the reagent, taking the wrong sample, or omitting a process.
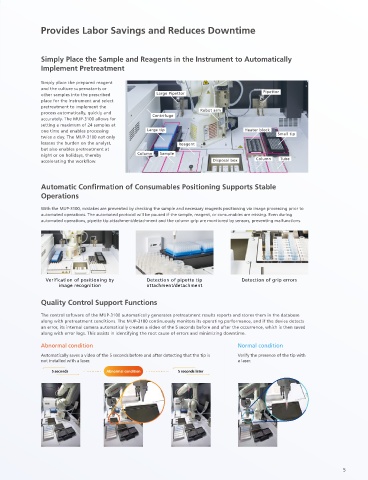
Automatic Confirmation of Consumables Positioning Supports Stable
Operations
Automation of the Entire Process
With the MUP-3100, mistakes are prevented by checking the sample and necessary reagents positioning via image processing prior to
automated operations. The automated protocol will be paused if the sample, reagent, or consumables are missing. Even during
automated operations, pipette tip attachment/detachment and the column grip are monitored by sensors, preventing malfunctions.
Culture solution
(including partially purified samples)
Addition of sample
Antibodies are captured
and purified by the
solid phase column
Verification of positioning by Detection of pipette tip Detection of grip errors
image recognition attachment/detachment
Centrifugation
Quality Control Support Functions
Addition of rinsing solution and centrifugation
The control software of the MUP-3100 automatically generates pretreatment results reports and stores them in the database
Addition of reaction accelerating agent and
Glycans are released centrifugation along with pretreatment conditions. The MUP-3100 continuously monitors its operating performance, and if the device detects
from the antibodies an error, its internal camera automatically creates a video of the 5 seconds before and after the occurrence, which is then saved
Addition of enzyme solution
along with error logs. This assists in identifying the root cause of errors and minimizing downtime.
Heating Abnormal condition Normal condition
Automatically saves a video of the 5 seconds before and after detecting that the tip is Verify the presence of the tip with
Addition of labeling reagent and centrifugation not installed with a laser. a laser.
Heating
5 seconds 5 seconds later
Fluorescent labeling
of the glycans
Detachment of antibody trap column
Addition of solvent
Labeled glycans are trapped by the column
Recovery of the Completion of preparation for labeled glycans
labeled glycans
4 5