Page 74 - Pharmaceutical Solution for Pharma Analysis
P. 74
Application No.J99"
News
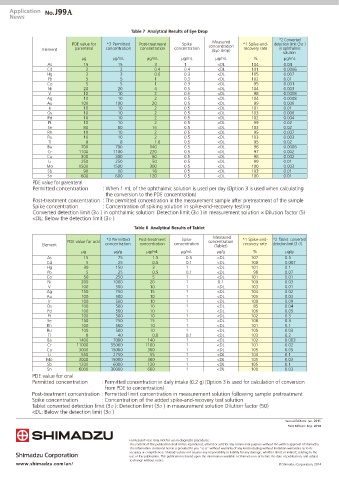
Table 7 Analytical Results of Eye Drop
*2 Converted
Measured
PDE value for *3 Permitted Post-treatment Spike concentration *1 Spike-and- detection limit (3М)
Element parenteral concentration concentration concentration (Eye drop) recovery rate in ophthalmic
solution
μg μg/mL μg/mL μg/mL μg/mL % μg/mL
As 15 15 3 1 <DL 104 0.04
Cd 2 2 0.4 0.4 <DL 101 0.0006
Hg 3 3 0.6 0.3 <DL 105 0.007
Pb 5 5 1 0.3 <DL 102 0.01
Co 5 5 1 0.3 <DL 95 0.001
Ni 20 20 4 0.5 <DL 104 0.003
V 10 10 2 0.5 <DL 98 0.0008
Ag 10 10 2 0.5 <DL 104 0.0008
Au 100 100 20 0.5 <DL 99 0.006
Ir 10 10 2 0.5 <DL 101 0.01
Os 10 10 2 0.5 <DL 103 0.006
Pd 10 10 2 0.5 <DL 102 0.004
Pt 10 10 2 0.5 <DL 99 0.02
Se 80 80 16 0.5 <DL 103 0.02
Rh 10 10 2 0.5 <DL 95 0.007
Ru 10 10 2 0.5 <DL 103 0.003
Tl 8 8 1.6 0.5 <DL 95 0.02
Ba 700 700 140 0.5 <DL 96 0.0006
Cr 1100 1100 220 0.5 <DL 97 0.002
Cu 300 300 60 0.5 <DL 96 0.002
Li 250 250 50 0.5 <DL 99 0.01
Mo 1500 1500 300 0.5 <DL 100 0.003
Sb 90 90 18 0.5 <DL 103 0.01
Sn 600 600 120 0.5 <DL 100 0.01
PDE value for parenteral
Permitted concentration : When 1 mL of the ophthalmic solution is used per day (Option 3 is used when calculating
the conversion to the PDE concentration)
Post-treatment concentration : The permitted concentration in the measurement sample after pretreatment of the sample
Spike concentration : Concentration of spiking solution in spike-and-recovery testing
Converted detection limit (3М) in ophthalmic solution: Detection limit (3М) in measurement solution × Dilution factor (5)
<DL: Below the detection limit (3М)
Table 8 Analytical Results of Tablet
Measured
*3 Permitted Post-treatment Spike *1 Spike-and- *2 Tablet converted
PDE value for oral concentration
Element concentration concentration concentration (Tablet) recovery rate detection limit (3М
μg μg/g μg/mL μg/mL μg/g % μg/g
As 15 75 1.5 0.5 <DL 107 0.5
Cd 5 25 0.5 0.1 <DL 100 0.007
Hg 30 150 3 1 <DL 101 0.1
Pb 5 25 0.5 0.1 <DL 98 0.07
Co 50 250 5 1 <DL 101 0.01
Ni 200 1000 20 1 0.1 100 0.03
V 100 500 10 1 <DL 103 0.01
Ag 150 750 15 1 <DL 104 0.02
Au 100 500 10 1 <DL 105 0.03
Ir 100 500 10 1 <DL 100 0.09
Os 100 500 10 1 <DL 85 0.04
Pd 100 500 10 1 <DL 106 0.05
Pt 100 500 10 1 <DL 102 0.3
Se 150 750 15 1 <DL 108 0.3
Rh 100 500 10 1 <DL 101 0.1
Ru 100 500 10 1 <DL 100 0.03
Tl 8 40 0.8 0.1 <DL 103 0.2
Ba 1400 7000 140 1 <DL 102 0.003
Cr 11000 55000 1100 1 <DL 101 0.02
Cu 3000 15000 300 1 <DL 105 0.05
Li 550 2750 55 1 <DL 104 0.1
Mo 3000 15000 300 1 <DL 101 0.03
Sb 1200 6000 120 1 <DL 105 0.1
Sn 6000 30000 600 1 <DL 100 0.03
PDE value for oral
Permitted concentration : Permitted concentration in daily intake (0.2 g) (Option 3 is used for calculation of conversion
from PDE to concentration)
Post-treatment concentration : Permitted limit concentration in measurement solution following sample pretreatment
Spike concentration : Concentration of the added spike-and-recovery test solution
Tablet converted detection limit (3М): Detection limit (3М) in measurement solution Dilution factor (50)
<DL: Below the detection limit (3М)
4FDPOE Edition: +VO. 201
First Edition: Sep. 2014
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2014