Page 46 - Pharmaceutical Solution for Pharma Analysis
P. 46
LAAN-A-GC-E052
Application Gas Chromatography
News
System Suitability Testing for
Hydroxypropyl Cellulose
No. G287
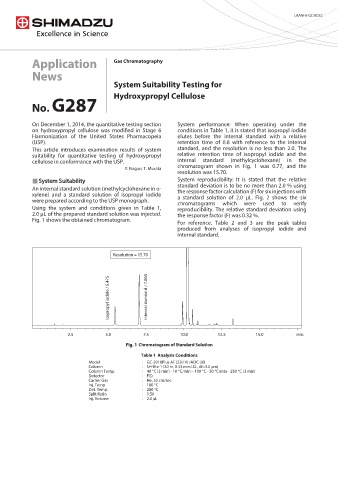
On December 1, 2014, the quantitative testing section System performance: When operating under the
on hydroxypropyl cellulose was modified in Stage 6 conditions in Table 1, it is stated that isopropyl iodide
Harmonization of the United States Pharmacopeia elutes before the internal standard with a relative
(USP). retention time of 0.8 with reference to the internal
This article introduces examination results of system standard, and the resolution is no less than 2.0. The
suitability for quantitative testing of hydroxypropyl relative retention time of isopropyl iodide and the
cellulose in conformance with the USP. internal standard (methylcyclohexane) in the
chromatogram shown in Fig. 1 was 0.77, and the
Y. Nagao, T. Murata
resolution was 15.70.
System Suitability System reproducibility: It is stated that the relative
An internal standard solution (methylcyclohexane in o- standard deviation is to be no more than 2.0 % using
the response factor calculation (F) for six injections with
xylene) and a standard solution of isopropyl iodide
were prepared according to the USP monograph. a standard solution of 2.0 μL. Fig. 2 shows the six
chromatograms which were used to verify
Using the system and conditions given in Table 1, reproducibility. The relative standard deviation using
2.0 μL of the prepared standard solution was injected. the response factor (F) was 0.32 %.
Fig. 1 shows the obtained chromatogram.
For reference, Table 2 and 3 are the peak tables
produced from analyses of isopropyl iodide and
internal standard.
Resolution = 15.70
Isopropyl iodide / 5.475 Internal standard / 7.063
2.5 5.0 7.5 10.0 12.5 15.0 min
Chromatogram of Standard Solution
Table 1 Analysis Conditions
Model : GC-2010Plus AF (230 V) /AOC-20i
Column : SH-Rtx-1 (30 m, 0.53 mm I.D., df=3.0 μm)
Column Temp. : 40 qC (3 min) - 10 qC/min – 100 qC - 50 qC/min - 250 qC (3 min)
Detector : FID
Carrier Gas : He, 52 cm/sec
Inj. Temp. : 180 qC
Det. Temp. : 280 qC
Split Ratio : 1:50
Inj. Volume : 2.0 μL