Page 68 - Clinical-Medical Solution for Clinical Chemistry
P. 68
Application No. C158
News
Structural Analysis Using In-Source CID
Analysis
While a triple quadrupole mass spectrometer is usually
used for structural analysis, a single quadrupole mass
spectrometer can also obtain molecular structure
information as well as molecular weight information by
setting the lens system to a high voltage.
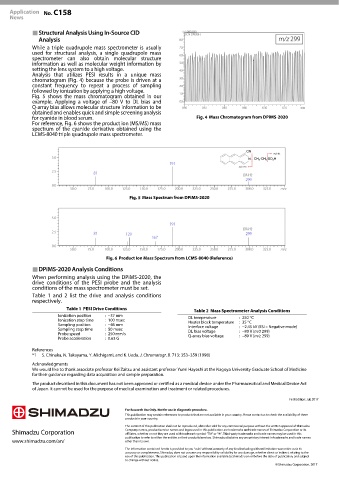
Analysis that utilizes PESI results in a unique mass
chromatogram (Fig. 4) because the probe is driven at a
constant frequency to repeat a process of sampling
followed by ionization by applying a high voltage.
Fig. 5 shows the mass chromatogram obtained in our
example. Applying a voltage of −80 V to DL bias and
Q-array bias allows molecular structure information to be
obtained and enables quick and simple screening analysis
for cyanide in blood serum. Mass Chromatogram from DPiMS-2020
For reference, Fig. 6 shows the product ion (MS/MS) mass
spectrum of the cyanide derivative obtained using the
LCMS-8040 triple quadrupole mass spectrometer.
Mass Spectrum from DPiMS-2020
Product Ion Mass Spectrum from LCMS-8040 (Reference)
DPiMS-2020 Analysis Conditions
When performing analysis using the DPiMS-2020, the
drive conditions of the PESI probe and the analysis
conditions of the mass spectrometer must be set.
Table 1 and 2 list the drive and analysis conditions
respectively.
Table 1 PESI Drive Conditions
Table 2 Mass Spectrometer Analysis Conditions
Ionization position : −37 mm
Ionization stop time : 100 msec DL temperature : 250 °C
: 35 °C
Heater block temperature
Sampling position : −46 mm Interface voltage : −2.45 kV (ESI – Negative mode)
Sampling stop time : 50 msec
Probe speed : 250 mm/s DL bias voltage : : −80 V (m/z 299)
−80 V (m/z 299)
Q-array bias voltage
Probe acceleration : 0.63 G
References
*1 S. Chinaka, N. Takayama, Y. Michigami, and K. Ueda. J. Chromatogr. B. 713: 353–359 (1998)
Acknowledgments
We would like to thank associate professor Kei Zaitsu and assistant professor Yumi Hayashi at the Nagoya University Graduate School of Medicine
for their guidance regarding data acquisition and sample preparation.
The product described in this document has not been approved or certified as a medical device under the Pharmaceutical and Medical Device Act
of Japan. It cannot be used for the purpose of medical examination and treatment or related procedures.
First Edition: Jul. 2017
For Research Use Only. Not for use in diagnostic procedure.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
Company names, product/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation or its
affiliates, whether or not they are used with trademark symbol “TM” or “”. Third-party trademarks and trade names may be used in this
publication to refer to either the entities or their products/services. Shimadzu disclaims any proprietary interest in trademarks and trade names
www.shimadzu.com/an/ other than its own.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
© Shimadzu Corporation, 2017