Page 8 - Clinical-Medical Physical Properties of Biomaterial
P. 8
Evaluation of Biomaterials To ensure that implant screws do not become loose inside the body, it is important to perform endurance testing of the fixing force.
Testing Implant Screws
Evaluation of Biological Samples Intended for transplantation, mainly into humans, biomaterials are biomaterials extends from static tensile testing, compression and perpendicular to the tightening axis and the changes in fixing forces are measured with respect to the number of vibrations. Evaluation of Biological Samples
Test jigs can test the screw loosening process under a variety of conditions. Vibration displacements are applied in the direction along
As bone screws for implants can break when inserted during surgery or during the trial period, it is important to
evaluate the mechanical properties of the bone screws.
testing, and dynamic testing of constituent metals and alloys and
used in both the medical and dental fields. Many types of material
Testing of the mechanical properties of the bone screws includes torsional fracture testing, which applies torque
compression and bending testing of entire devices, to fatigue
are used, including ceramics and metals for artificial joints, dental
at a constant rotational speed until the screw fails; screw-in testing by screwing bone screws at constant force
testing and pressure pulsation simulations of stent materials.
implants, and artificial bone; polymers for artificial blood vessels;
and constant speed into simulated bone to evaluate the screw-in properties; and pull-out testing, which pulls
and stents combining metal wires in mesh to locally expand tubular
Some biomaterial testing applications for bone implants, dental
screws out of simulated bone at a constant rate to evaluate the fixation of the bone screws.
tissue such as blood vessels. Mechanical testing of these
screwdriver
c)
b)
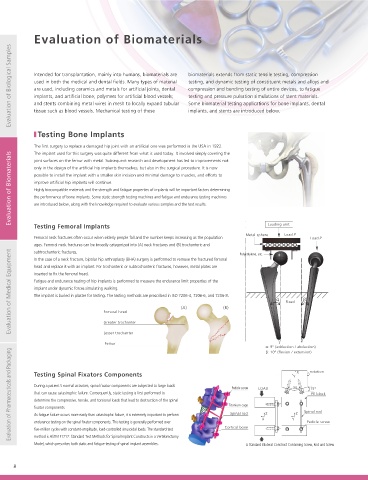
Testing Bone Implants implants, and stents are introduced below. a) Bone Bone screwdriver Pull-out force (F)
Simulated bone
5 threads
The first surgery to replace a damaged hip joint with an artificial one was performed in the USA in 1922. Fixture Fixture Fixture
Evaluation of Biomaterials only in the design of the artificial hip implants themselves, but also in the surgical procedure. It is now Torsional Testing of Screws b) Screw-In Test sample is measured when it is subjected to torsional, compressive, and Evaluation of Biomaterials
The implant used for this surgery was quite different from what is used today. It involved simply covering the
joint surfaces on the femur with metal. Subsequent research and development has led to improvements not
possible to install the implant with a smaller skin incision and minimal damage to muscles, and efforts to
a) Torsional Fracture Test
c) Pull-Out Test
improve artificial hip implants will continue.
Highly biocompatible materials and the strength and fatigue properties of implants will be important factors determining
the performance of bone implants. Some static strength testing machines and fatigue and endurance testing machines
are introduced below, along with the knowledge required to evaluate various samples and the test results.
This machine can be used for strength testing of screws used in bone
implants and dental implants and for materials testing of zirconia implant
sample, an essential process to ensure that implant materials do not break
material. (Samples are attached to the testing machine via an adapter after
Loading unit
Testing Femoral Implants
being processed into a special shape.) Screws and other specially shaped tensile test forces. The tester measures the mechanical properties of the
when screws are inserted and do not suffer fatigue failure during use.
Metal sphere Load P
Femoral neck fractures often occur when elderly people fall and the number keeps increasing as the population Load P samples are mounted in a 3-jaw scroll chuck. The torque applied to the
ages. Femoral neck fractures can be broadly categorized into (A) neck fractures and (B) trochanteric and
Torque cell Scroll chuck
Evaluation of Medical Equipment inserted to fix the femoral head. Femoral head (A) (B) α Fixed β Evaluation of Medical Equipment
subtrochanteric fractures.
Polyethylene, etc.
In the case of a neck fracture, bipolar hip arthroplasty (BHA) surgery is performed to remove the fractured femoral
Handle
head and replace it with an implant. For trochanteric or subtrochanteric fractures, however, metal plates are
Fatigue and endurance testing of hip implants is performed to measure the endurance limit properties of the
implant under dynamic forces simulating walking.
The implant is buried in plaster for testing. The testing methods are prescribed in ISO 7206-4, 7206-6, and 7206-8.
Greater trochanter
Load point
Lesser trochanter
Sample retainer
Load
Femur Retainer P
α: 9º (adduction / abduction) Sample Retainer Support roller Plate
Evaluation of Pharmaceuticals and Packaging Testing Spinal Fixators Components Titanium cage LOAD Z Y 30 Z X Spinal rod Testing Bolt Loosening Bending Testing of Bone Plates Evaluation of Pharmaceuticals and Packaging
β: 10º (flexion / extension)
Load roller
rotation
Y
Servopulser Fatigue and Endurance Tester
During a patient's normal activities, spinal fixator components are subjected to large loads
Pedicle screw
15°
Bone plates are screwed onto the bone surface to immobilize the fracture site. They can
that can cause catastrophic failure. Consequently, static testing is first performed to
PE block
To ensure that implant screws do not become loose inside the
be used as metal implants for fixation after limb bone or vertebrae fractures or in the
determine the compressive, tensile, and torsional loads that lead to destruction of the spinal
body, it is important to perform endurance testing of the
fixator components.
period before bone adhesion is achieved after corrective osteotomy.
Spinal rod
fixing force. Test jigs can test the screw loosening process
As fatigue failure occurs more easily than catastrophic failure, it is extremely important to perform
The mechanical properties of bone plates are evaluated by bending testing. The
X
endurance testing on the spinal fixator components. This testing is generally performed over
Pedicle screw
applied in the direction along and perpendicular to the
five-million cycles with constant-amplitude, load-controlled sinusoidal loads. The standard test
sample and support rollers that support the sample. They are positioned symmetrically
tightening axis and the changes in fixing forces are measured
method is ASTM F1717: Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy
and the spacing between them can be altered. The sample retainers ensure that the
with respect to the number of vibrations.
Model, which prescribes both static and fatigue testing of spinal implant assemblies. Cortical bone A Standard Bilateral Construct Containing Screw, Rod and Screw under a variety of conditions. Vibration displacements are three-point bending test jig is comprised of load rollers that apply test forces to the
sample cannot move during endurance testing.
Physical Property Testing Equipment
for Biomaterials and Medical Applications
8 Solutions for Biomedical Testing 9