Page 16 - Shimadzu Journal vol.10 Issue1
P. 16
Materials Science
Specimens
In this study, three different valence manganese oxides (MnO s),
x
MnO (II), Mn O (III), and MnO (IV), and two different valence
2 3 2
lithium manganese oxides (LiMnO s), LiMn O (III, IV) and
x 2 4
Li MnO (IV), were prepared for use in the valence identification
2 3
of Mn in LIB cathode materials. CoO (II) and NiO (II) as well as
LiCoO (III) and LiNiO (III) were prepared for use in the valence
2 2
identification of Co and Ni in LIB cathode materials. The use of
CoO and NiO was unavoidable because divalent Li oxides of Co
and Ni could not be obtained.
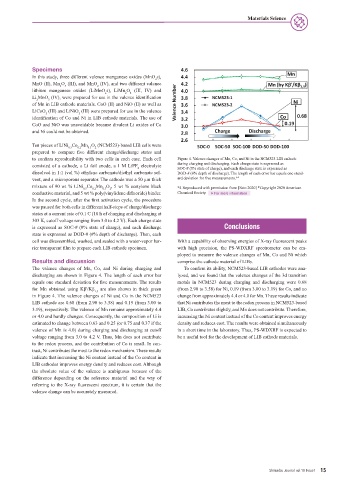
Ten pieces of LiNi Co Mn O (NCM523)-based LIB cells were
0.5 0.2 0.3 2
prepared to compare five different charge/discharge states and
to confirm reproducibility with two cells in each case. Each cell Figure 4. Valence changes of Mn, Co, and Ni in the NCM523 LIB cathode
consisted of a cathode, a Li foil anode, a 1 M LiPF electrolyte during charging and discharging. Each charge state is expressed as
6 SOC-# (#% state of charge), and each discharge state is expressed as
dissolved in 1:1 (vol %) ethylene carbonate/diethyl carbonate sol- DOD-# (#% depth of discharge). The length of each error bar equals one stand-
vent, and a microporous separator. The cathode was a 50 μm thick ard deviation for five measurements.* 4
mixture of 90 wt % LiNi Co Mn O , 5 wt % acetylene black
0.5 0.2 0.3 2 *4 Reproduced with permission from [Sato 2020] *Copyright 2020 American
conductive material, and 5 wt % poly(vinylidene difluoride) binder. Chemical Society. For more information
In the second cycle, after the first activation cycle, the procedure
was paused for both cells in different half-steps of charge/discharge
states at a current rate of 0.1 C (10 h of charging and discharging at
303 K, cutoff voltage ranging from 3.0 to 4.2 V). Each charge state
is expressed as SOC-# (#% state of charge), and each discharge Conclusions
state is expressed as DOD-# (#% depth of discharge). Then, each
cell was disassembled, washed, and sealed with a water-vapor bar- With a capability of observing energies of X-ray fluorescent peaks
rier transparent film to prepare each LIB cathode specimen. with high precision, the PS-WDXRF spectrometer can be em-
ployed to measure the valence changes of Mn, Co and Ni which
Results and discussion comprise the cathode material of LIBs.
The valence changes of Mn, Co, and Ni during charging and To confirm its ability, NCM523-based LIB cathodes were ana-
discharging are shown in Figure 4. The length of each error bar lyzed, and we found that the valence changes of the 3d transition
equals one standard deviation for five measurements. The results metals in NCM523 during charging and discharging were 0.68
for Mn obtained using Kβ′/Kβ are also shown in thick green (from 2.90 to 3.58) for Ni, 0.19 (from 3.00 to 3.19) for Co, and no
1,3
in Figure 4. The valence changes of Ni and Co in the NCM523 change from approximately 4.4 or 4.0 for Mn. These results indicate
LIB cathode are 0.68 (from 2.90 to 3.58) and 0.19 (from 3.00 to that Ni contributes the most to the redox process in NCM523-based
3.19), respectively. The valence of Mn remains approximately 4.4 LIB, Co contributes slightly, and Mn does not contribute. Therefore,
or 4.0 and hardly changes. Consequently, the composition of Li is increasing the Ni content instead of the Co content improves energy
estimated to change between 0.63 and 0.25 (or 0.75 and 0.37 if the density and reduces cost. The results were obtained simultaneously
valence of Mn is 4.0) during charging and discharging at cutoff in a short time in the laboratory. Thus, PS-WDXRF is expected to
voltage ranging from 3.0 to 4.2 V. Thus, Mn does not contribute be a useful tool for the development of LIB cathode materials.
to the redox process, and the contribution of Co is small. In con-
trast, Ni contributes the most to the redox mechanism. These results
indicate that increasing the Ni content instead of the Co content in
LIB cathodes improves energy density and reduces cost. Although
the absolute value of the valence is ambiguous because of the
difference depending on the reference material and the way of
referring to the X-ray fluorescent spectrum, it is certain that the
valence change can be accurately measured.
Shimadzu Journal vol.10 Issue1 15