Page 15 - Shimadzu Journal vol.10 Issue1
P. 15
Materials Science
Application to valence analysis
of LIB
Peak energy of fluorescent X-ray emitted from a ma-
terial shifts along with a change in the chemical state
of the element. Figure 2 shows an overlay of Mn Kβ
spectra of MnO s and LiMnO s normalized by peak in-
x x
tensity. The inset shows the enlarged view of the top of
Kβ . It is clear that the peak energy shifts according
1,3
to the nominal valence of Mn. In addition to that, the
change in the peak intensity of Kβ′ is also observed.
By plotting the peak energy as a function of nominal Figure 1. Schematic of PS-WDXRF. Fluorescent X-rays from the specimen pass through the slit
valence of Mn, it was found that the Mn Kβ peak and are dispersed by the flat analyzing crystal. They are simultaneously detected by different
1,3 channels of the detector corresponding to different energies. The rotation mechanism of the
energies of the MnO specimens are negatively propor- specimen eliminates the position dependence of the measurement point, and spatially averaged
x measurement data can be obtained.* 1
tional to the valence, and those of the LiMnO speci-
x
mens showed a similar relation, see Figure 3(a).
The dependence of the peak intensity ratio Kβ′/Kβ
1,3
on the nominal valence is shown in Figure 3(b), which
indicates that the peak intensity ratios of the MnO and
x
LiMnO specimens were also negatively proportional to
x
the valence and displayed a similar dependence as that
of the peak energy.
These suggest that the changes in the peak energy of
Mn Kβ and the peak intensity ratio Kβ′/Kβ can be
1,3 1,3
used for valence identification.
For other elements, Co, and Ni, out of three im-
portant elements in LIB cathode materials, it had been
reported that they also showed similar behavior in terms
of the relationship between the peak energy shift and
the nominal valence of the element. Figure 2. Overlay of the Mn Kβ spectra of MnO s and LiMnO s fitted by the Lorentz
x
x
function, normalized by peak intensity. The inset shows the enlarged view of the top
of Kβ . It is clear that the peak energy shifts, and the peak intensity of Kβ′ changes
1,3
according to the valence of Mn.* 2
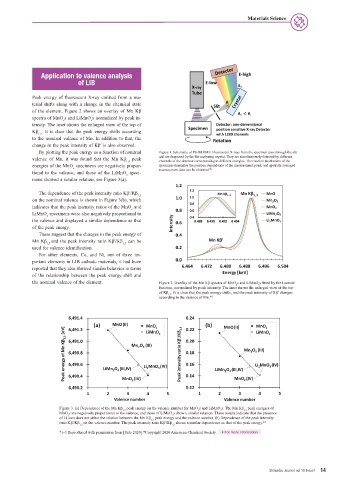
Figure 3. (a) Dependence of the Mn Kβ peak energy on the valence number for MnO s and LiMnO s. The Mn Kβ peak energies of
x
x
1,3
1,3
MnO s are negatively proportional to the valence, and those of LiMnO s show a similar relation. These results indicate that the presence
x
x
of Li ions does not affect the relation between the Mn Kβ peak energy and the valence number. (b) Dependence of the peak intensity
1,3
ratio Kβ′/Kβ on the valence number. The peak intensity ratio Kβ′/Kβ shows a similar dependence as that of the peak energy.* 3
1,3 1,3
*1-3 Reproduced with permission from [Sato 2020] *Copyright 2020 American Chemical Society. For more information
Shimadzu Journal vol.10 Issue1 14