Page 15 - Shimadzu Journal vol.8 Issue2
P. 15
Insight from customer / Report
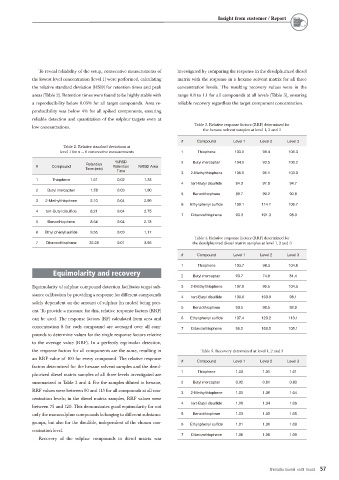
To reveal reliability of the setup, consecutive measurements of investigated by comparing the response in the desulphurized diesel
the lowest level concentration (level 1) were performed, calculating matrix with the response in a hexane solvent matrix for all three
the relative standard deviation (RSD) for retention times and peak concentration levels. The resulting recovery values were in the
areas (Table 2). Retention times were found to be highly stable with range 0.8 to 1.1 for all compounds at all levels (Table 5), ensuring
a reproducibility below 0.05% for all target compounds. Area re- reliable recovery regardless the target component concentration.
producibility was below 4% for all spiked components, ensuring
reliable detection and quantitation of the sulphur targets even at
Table 3. Relative response factors (RRF) determined for
low concentrations.
the hexane solvent samples at level 1, 2 and 3
# Compound Level 1 Level 2 Level 3
Table 2. Relative standard deviations at
level 1 for n = 6 consecutive measurements 1 Thiophene 103.5 98.4 106.3
%RSD 2 Butyl mercaptan 104.5 93.5 100.2
Retention
# Compound Retention %RSD Area
Time (min)
Time
3 2-Methylthiophene 105.5 95.1 103.0
1 Thiophene 1.61 0.02 1.33
4 tert-Butyl disulfide 94.3 97.8 94.7
2 Butyl mercaptan 1.78 0.03 1.90
5 Benzothiophene 89.7 99.2 90.8
3 2-Methylthiophene 2.10 0.04 2.99
6 Ethyl phenyl sulfide 109.1 114.7 106.7
4 tert-Butyl disulfide 8.21 0.04 2.75
7 Dibenzothiophene 93.3 101.3 98.3
5 Benzothiophene 8.54 0.04 2.78
6 Ethyl phenyl sulfide 9.55 0.03 1.17
Table 4. Relative response factors (RRF) determined for
7 Dibenzothiophene 22.28 0.01 3.93 the desulphurized diesel matrix samples at level 1, 2 and 3
# Compound Level 1 Level 2 Level 3
1 Thiophene 103.7 98.3 104.8
Equimolarity and recovery
2 Butyl mercaptan 93.7 74.8 81.4
Equimolarity of sulphur compound detection facilitates target sub- 3 2-Methylthiophene 107.9 99.5 104.5
stance calibration by providing a response for different compounds
4 tert-Butyl disulfide 100.6 100.9 98.1
solely dependent on the amount of sulphur (in moles) being pres-
5 Benzothiophene 90.5 98.5 92.9
ent. To provide a measure for this, relative response factors (RRF)
can be used. The response factors (RF) calculated from area and 6 Ethyl phenyl sulfide 107.4 120.2 113.1
concentration S for each compound are averaged over all com-
7 Dibenzothiophene 96.2 108.0 105.1
pounds to determine values for the single response factors relative
to the average value (RRF). In a perfectly equimolar detection,
the response factors for all components are the same, resulting in Table 5. Recovery determined at level 1, 2 and 3
an RRF value of 100 for every compound. The relative response
# Compound Level 1 Level 2 Level 3
factors determined for the hexane solvent samples and the desul-
1 Thiophene 1.03 1.01 1.01
phurized diesel matrix samples of all three levels investigated are
summarized in Table 3 and 4. For the samples diluted in hexane, 2 Butyl mercaptan 0.92 0.81 0.83
RRF values were between 90 and 115 for all compounds at all con-
3 2-Methylthiophene 1.05 1.06 1.04
centration levels; in the diesel matrix samples, RRF values were
4 tert-Butyl disulfide 1.09 1.04 1.06
between 75 and 120. This demonstrates good equimolarity for not
only the monosulphur compounds belonging to different substance 5 Benzothiophene 1.03 1.00 1.05
groups, but also for the disulfide, independent of the chosen con-
6 Ethyl phenyl sulfide 1.01 1.06 1.08
centration level.
7 Dibenzothiophene 1.06 1.08 1.09
Recovery of the sulphur compounds in diesel matrix was
Shimadzu Journal vol.8 Issue2 57