Page 29 - Shimadzu Journal vol.7 Issue1
P. 29
Environmental Analysis
⿏PFAS Stability Study – Effects of Solvents, LC Vial ⿏Calibration Range and Method Detection Limit (MDL)
Materials and Vortex
Calibration was performed for all PFAS compounds using a nine-point
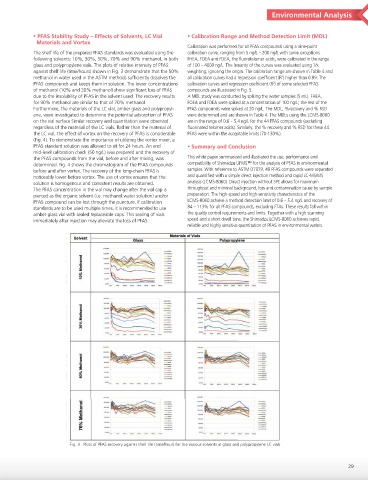
The shelf life of the prepared PFAS standards was evaluated using the calibration curve, ranging from 5 ng/L – 200 ng/L with some exceptions.
following solvents: 10%, 30%, 50%, 70% and 90% methanol, in both FHEA, FOEA and FDEA, the fluorotelomer acids, were calibrated in the range
glass and polypropylene vials. The plots of relative intensity of PFAS of 100 – 4000 ng/L. The linearity of the curves was evaluated using 1/x
against shelf life (time/hours) shown in Fig. 3 demonstrate that the 50% weighting, ignoring the origin. The calibration range are shown in Table 4 and
2
methanol in water used in the ASTM methods sufficiently dissolves the all calibration curves had a regression coefficient (R ) higher than 0.99. The
2
PFAS compounds and keeps them in solution. The lower concentrations calibration curves and regression coefficient (R ) of some selected PFAS
of methanol (10% and 30% methanol) show significant loss of PFAS compounds are illustrated in Fig. 5.
due to the insolubility of PFAS in the solvent used. The recovery results A MDL study was conducted by spiking the water samples (5 mL). FHEA,
for 90% methanol are similar to that of 70% methanol. FOEA and FDEA were spiked at a concentration of 100 ng/L; the rest of the
Furthermore, the materials of the LC vial, amber glass and polypropyl- PFAS compounds were spiked at 20 ng/L. The MDL, %recovery and % RSD
ene, were investigated to determine the potential adsorption of PFAS were determined and are shown in Table 4. The MDLs using the LCMS-8060
on the vial surface Similar recovery and quantitation were observed are in the range of 0.6 – 5.4 ng/L for the 44 PFAS compounds (excluding
regardless of the material of the LC vials. Rather than the material of fluorinated telomer acids). Similarly, the % recovery and % RSD for these 44
the LC vial, the effect of vortex on the recovery of PFAS is considerable PFAS were within the acceptable limits (70-130%).
(Fig. 4). To demonstrate the importance of utilizing the vortex mixer, a
PFAS standard solution was allowed to sit for 24 hours. An end ⿏Summary and Conclusion
mid-level calibration check (50 ng/L) was prepared and the recovery of
the PFAS compounds from the vial, before and after mixing, was This white paper summarized and illustrated the use, performance and
determined. Fig. 4 shows the chromatogram of the PFAS compounds compatibility of Shimadzu UFMS™ for the analysis of PFAS in environmental
before and after vortex. The recovery of the long-chain PFAS is samples. With reference to ASTM D7979, 49 PFAS compounds were separated
noticeably lower before vortex. The use of vortex ensures that the and quantified with a simple direct injection method and rapid LC-MS/MS
solution is homogenous and consistent results are obtained. analysis (LCMS-8060). Direct injection without SPE allows for maximum
The PFAS concentration in the vial may change after the vial cap is throughput and minimal background, loss and contamination cause by sample
pierced as the organic solvent (i.e. methanol:water solution) and/or preparation. The high-speed and high-sensitivity characteristics of the
PFAS compound can be lost through the puncture. If calibration LCMS-8060 achieve a method detection limit of 0.6 – 5.4 ng/L and recovery of
standards are to be used multiple times, it is recommended to use 84 – 113% for all PFAS compounds, excluding FTAs. These results fall within
amber glass vial with sealed replaceable caps. This sealing of vials the quality control requirements and limits. Together with a high scanning
immediately after injection may alleviate the loss of PFAS. speed and a short dwell time, the Shimadzu LCMS-8060 achieves rapid,
reliable and highly sensitive quantitation of PFAS in environmental waters.
Fig. 3 Plots of PFAS recovery against shelf life (time/hour) for the various solvents in glass and polypropylene LC vials
29