Page 27 - Shimadzu Journal vol.7 Issue1
P. 27
Environmental Analysis
The described LC-MS/MS method was run exactly as indicated in ASTM of PFAS during and, at best, minimize them with the use of PFAS-free
Method D7979. One such modification concerns the ASTM liquid materials, high-grade solvents and flushing the instrument by injecting
chromatography (LC) conditions. Only two LC mobile phases were multiple method blanks.
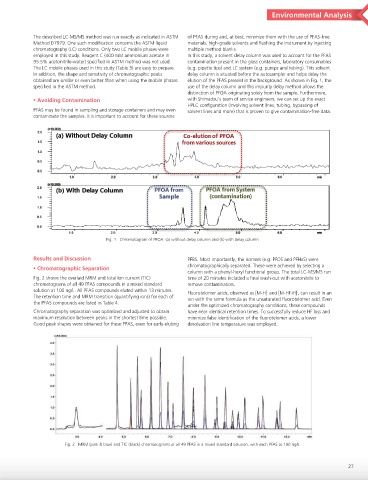
employed in this study. Reagent C (400 mM ammonium acetate in In this study, a solvent delay column was used to account for the PFAS
95:5% acetonitrile-water) specified in ASTM method was not used. contamination present in the glass containers, laboratory consumables
The LC mobile phases used in this study (Table 3) are easy to prepare. (e.g. pipette tips) and LC system (e.g. pumps and tubing). This solvent
In addition, the shape and sensitivity of chromatographic peaks delay column is situated before the autosampler and helps delay the
obtained are similar or even better than when using the mobile phases elution of the PFAS present in the background. As shown in Fig. 1, the
specified in the ASTM method. use of the delay column and this impurity delay method allows the
distinction of PFOA originating solely from the sample. Furthermore,
⿏Avoiding Contamination with Shimadzu’s team of service engineers, we can set up the exact
HPLC configuration (involving solvent lines, tubing, bypassing of
PFAS may be found in sampling and storage containers and may even solvent lines and more) that is proven to give contamination-free data.
contaminate the samples. It is important to account for these sources
Fig. 1 Chromatogram of PFOA: (a) without delay column and (b) with delay column
Results and Discussion PFBS. Most importantly, the isomers (e.g. PFOS and PFHxS) were
chromatographically separated. These were achieved by selecting a
⿏Chromatographic Separation
column with a phenyl-hexyl functional group. The total LC-MS/MS run
Fig. 2 shows the overlaid MRM and total ion current (TIC) time of 20 minutes included a final wash-out with acetonitrile to
chromatograms of all 49 PFAS compounds in a mixed standard remove contamination.
solution at 100 ng/L. All PFAS compounds eluted within 13 minutes. Fluorotelomer acids, observed as [M-H] and [M-HF-H] , can result in an
-
-
The retention time and MRM transition (quantifying ions) for each of ion with the same formula as the unsaturated fluorotelomer acid. Even
the PFAS compounds are listed in Table 4.
under the optimized chromatography conditions, these compounds
Chromatography separation was optimized and adjusted to obtain have near identical retention times. To successfully reduce HF loss and
maximum resolution between peaks in the shortest time possible. minimize false identification of the fluorotelomer acids, a lower
Good peak shapes were obtained for these PFAS, even for early-eluting desolvation line temperature was employed.
Fig. 2 MRM (pink & blue) and TIC (black) chromatograms of all 49 PFAS in a mixed standard solution, with each PFAS at 100 ng/L
27