Page 19 - Shimadzu Journal vol.2 Issue1
P. 19
Technical Report Technical Report
2-2. Simultaneous Quantitation of Main Area (×10 ) 6 3. Summary of i-DReC Settings 2. A mark indicating whether i-DReC has been applied, the
Component and Minor Impurities wavelength used for correction, the retention time of the
30 Correlation factor i-DReC parameters are set as part of the data processing spectrum used for sensitivity correction, and the calculated
This section demonstrates the use of i-DReC to simultaneously R=0.9996726 parameters for the photodiode array detector and can be applied sensitivity correction factor can be shown in the peak table and
quantify a relatively high concentration major component in a 25 in data processing methods for routine analysis without requiring compound table.
pharmaceutical sample and the relatively low concentration impurities. post-run operations. The following is a summary of parameters
Samples in which the concentration of the main component ranged 20 and their descriptions.
from 0.01 g/L to 1 g/L where analyzed using the SPD-M30A Correction Item Description
by i-DReC
photodiode array detector equipped with the high sensitivity cell. Fig. 15 1. i-DReC parameters are set in the multi chromatogram table. C = peaks to which i-DReC was applied
4 shows the calibration curve for the main component based on peak Mark E1-E4 = error in i-DReC calculation
area in the extracted chromatogram at 250 nm. For peaks to which i-DReC was applied,
10 DRE wavelength
Original this is the wavelength used for correction.
Analytical Conditions 5 For peaks to which i-DReC was applied,
Pump : Shimadzu LC-30AD×2 DRE factor this is the absorption ratio used for
sensitivity correction.
Detection : Shimadzu SPD-M30A
Column oven : Shimadzu CTO-20AC 0 DRE time For peaks to which i-DReC was applied,
Controller : Shimadzu CBM-20Alite 0 0.25 0.5 0.75 1 this is the retention time of the spectrum
Autosampler : Shimadzu SIL-30AC Conc. (g/L) used for sensitivity correction.
Column : Shimadzu Shim-pack XR-ODS (a) Calibration curve of the main component
(150 mmL. × 3.0 mmI.D., 2.2 µm)
Mobile phase A : 5% MeCN + 0.05% TFA i-DReC requires that spectrum similarity is maintained across the
Mobile phase B : 95% MeCN + 0.05% TFA peak. When peak separation is insufficient, i-DReC may not be
Time program : 2% (0–1.2 min) 2–98% (1.2–8.9 min) Error (%)
98% (8.9–10.8 min) 98–2% (10.8–11.1 min) 4 able to be applied.
STOP (14 min)
Flow rate : 1 mL/min 2
Column temp. : 40 °C 3. The peak area and height corrected by i-DReC can be used in
Sampling : 160 msec normal quantitation processes. The simple implementation allows
Slit width : 8 nm 0 0 0.25 0.5 0.75 1 the seamless use of i-DReC for routine analysis.
Time constant : 160 msec Parameter Description
Wavelength range : 190 nm-700 nm -2
Cell light path : 85 mm Dynamic range extension Select whether i-DReC is applied or not.
Injection volume : 1 µL -4 When intensity at peak top is over the 4. Conclusion
Threshold threshold, i-DReC is applied to the peak
(b) Error of corrected calibration points for correction. i-DReC's ability to calculate corrected peak area and height for
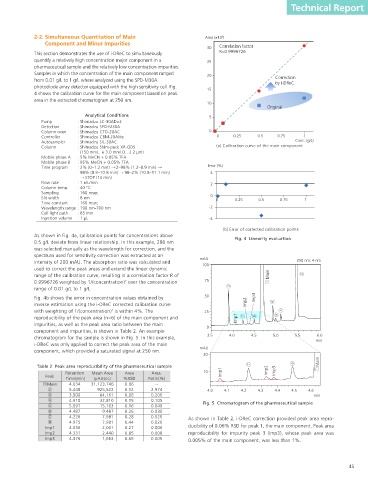
As shown in Fig. 4a, calibration points for concentrations above Fig. 4 Linearity evaluation Wavelength for correction Select whether wavelength for correction high concentration samples is made possible by the improved
0.5 g/L deviate from linear relationship. In this example, 280 nm (manual/auto) will be set manually or automatically. performance of the SPD-M30A photodiode array detector and the
was selected manually as the wavelength for correction, and the When "Manual" is selected, set the excellent reproducibility of the Nexera X2 system. The key features
spectrum used for sensitivity correction was extracted at an mAU Ch# channel number of the chromatogram of i-DReC can be summarized as follows.
intensity of 200 mAU. The absorption ratio was calculated and 100 250 nm, 4 nm that will be used.
used to correct the peak areas and extend the linear dynamic Intensity for When "Auto" is selected, set the target
range of the calibration curve, resulting in a correlation factor R of Main correction wavelength intensity for determining an appropriate Extension of the linear dynamic range using spectral similarity.
correction wavelength.
0.9996726 weighted by 1/(concentration) over the concentration 75 When "Auto" is selected, set the direction Simultaneous quantitation of both low and high concentration
2
range of 0.01 g/L to 1 g/L. Direction (+/-) to search for an appropriate correction compounds in a single injection.
Fig. 4b shows the error in concentration values obtained by 50 Imp3 wavelength. (+ = longer wavelength, Requires the use of only one PDA detector.
inverse estimation using the i-DReC corrected calibration curve Imp2 – = shorter wavelength) Standard samples are not necessary for correction.
2
with weighting of 1/(concentration) is within 4%. The 25 Intensity to extract Set intensity for extraction of sensitivity
correction spectrum.
reproducibility of the peak area (n=6) of the main component and Imp1 Select whether background compensation Simple method settings allow the use of i-DReC in routine analysis.
impurities, as well as the peak area ratio between the main 0 Background compensation is used or not for sensitivity correction i-DReC can be applied to samples containing a wide range of
component and impurities, is shown in Table 2. An example spectrum. compound concentrations. The use of i-DReC improves the
6.0
chromatogram for the sample is shown in Fig. 5. In this example, 3.5 4.0 4.5 5.0 5.5 min efficiency of sample pretreatment processes and laboratory
i-DReC was only applied to correct the peak area of the main The concentration range over which i-DReC effectively extends the productivity.
component, which provided a saturated signal at 250 nm. mAU
20 linear dynamic range is dependent upon the shape of the spectrum
Main of target peaks. The lower the slope of the spectrum around the
Table 2 Peak area reproducibility of the pharmaceutical sample correction wavelength, the more reliable the correction.
Retention Mean Area Area Area 10 Imp1 Imp2 Imp3
Peak Time(min) (µAUsec) %RSD Ratio(%)
4.634 31,123,746 0.06 --
5.448 925,522 0.12 2.974 4.0 4.1 4.2 4.3 4.4 4.5 4.6
3.900 64,161 0.08 0.206 min
4.910 32,810 0.15 0.105
5.091 15,103 0.16 0.049 Fig. 5 Chromatogram of the pharmaceutical sample
4.487 9,487 0.26 0.030
4.226 7,981 0.28 0.026 As shown in Table 2, i-DReC correction provided peak area repro-
4.975 7,981 0.44 0.026
4.056 2,001 0.27 0.006 ducibility of 0.06% RSD for peak 1, the main component. Peak area
4.331 2,440 0.85 0.008 reproducibility for impurity peak 3 (Imp3), whose peak area was
4.376 1,663 0.65 0.005 0.005% of the main component, was less than 1%.
45 46