Page 12 - Shimadzu Journal vol.1
P. 12
͠
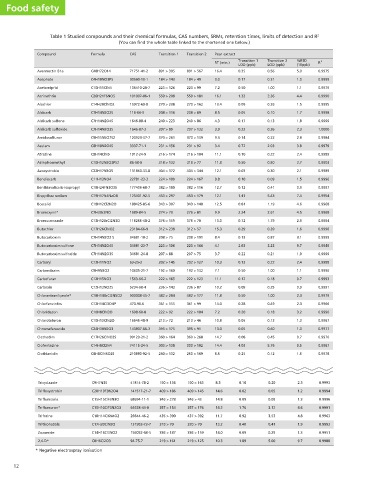
0.9996 0.9994 0.9936 0.9997 0.9991 0.9997 0.9994 0.9980 0.9971 0.9910 0.9946 0.9998 0.9994 0.9999 0.9999 0.9977 0.9983 0.9988 0.9970 0.9961 0.9976 0.9964 0.9944 0.9989 0.9995 0.9997 0.9985 0.9974 0.9998 0.9983 0.9994 0.9974 0.9986 0.9989 0.9996 0.9967 0.9987 0.9993 0.9984 0.9985 1.0000 0.9947 0.9968 0.9999 0.9982 0.9956
R 2
%RSD (10ppb) 2.2 2.6 9.2 1.6 2.5 0.5 2.9 2.6 0.6 2.5 1.7 2.5 0.7 0.9 0.9 1.8 3.1 1.9 1.9 4.3 3.3 5.0 0.9 2.4 1.6 1.2 1.5 9.8 4.1 7.1 2.7 4.2 1.6 8.2 1.3 2.1 1.7 5.7 3.2 2.7 1.8 1.7 3.1 13.4 4.7 4.5
Transition 2 LOD (ppb) 0.12 0.53 7.48 0.07 0.41 0.14 0.22 0.15 0.26 2.00 0.54 0.37 0.59 0.16 0.02 0.24 0.06 0.18 0.28 0.25 0.52 0.40 0.95 0.20 0.13 0.17 0.27 3.61 0.35 3.61 0.27 1.00 0.06 0.63 0.14 0.17 0.22 1.75 0.43 0.12 0.13 0.05 0.96 6.86 1.36 1.02
Transition 1 LOD (ppb) 0.06 0.18 2.21 0.05 0.29 0.12 0.10 0.05 0.09 1.00 0.30 0.12 0.18 0.02 0.02 0.14 0.03 0.06 0.05 0.31 0.18 0.23 0.75 0.10 0.05 0.02 0.18 3.75 0.11 0.55 0.20 1.00 0.04 0.24 0.12 0.05 0.19 1.13 0.29 0.05 0.07 0.05 0.30 6.20 0.15 2.25
Pear extract RT (min.) 12.2 14.5 13.5 7.0 12.7 13.7 3.9 10.8 11.4 4.8 13.5 13.3 10.6 6.2 6.5 10.8 16.9 12.4 13.5 10.2 10.0 13.4 13.1 13.6 14.1 15.9 10.1 10.4 13.5 11.8 15.2 12.4 13.2 15.7 10.6 12.7 13.1 7.8 11.1 10.7 15.1 12.3 11.5 13.3 11.4 15.6
Table 1 Continued... Transition 2 Transition 1 268 > 124 268 > 226 406 > 188 406 > 251 311 > 141 311 > 158 230 > 199 230 > 125 388 > 165 388 > 301 327 > 116 327 > 205 203 > 157 203 > 129 291 > 97 291 > 213 235 > 72 233 > 72 163 > 122 163 > 107 228 > 60 228 > 71 330 > 101 330 > 121 226 > 169 226 > 107 275 > 201 275 > 107 242 > 185 242 > 107 210 > 98 210 > 140 394 > 359 394 > 177 312 > 236 312 > 92 304 > 202 304 > 217 336 > 188 336 > 266 320 > 171 320 > 108 337 > 70 337 > 125 302 > 55 302 > 97 302 > 116 302 > 88 304 > 117 304 > 147 ࠓճඞཁແ 422 > 215 422 > 366 295 > 280 295 > 109 311 > 125 311 > 109 435 > 250 435 > 330 328 > 91 328 > 282 463 > 398 463 > 416 247 > 180 247 > 126 3
87130-20-9 119446-68-3 35367-38-5 110488-70-5 149961-52-4 165252-70-0 2497-07-6 330-54-1 33089-74-6 2439-10-3 135319-73-2 29973-13-5 53380-23-7 53380-22-6 23947-60-6 80844-07-1 161326-34-7 22224-92-6 31972-44-8 31972-43-7 114369-43-6 126833-17-8 79127-80-3 67564-91-4 111812-58-9 3761-41-9 3761-42-0 120068-37-3 69335-91-7 79622-59-6 131341-86-1 142459-58-3 101463-69-8 2164-17-2 239110-15-7 361377-29-9 69377-81-7 76674-21-0 98886-44-3 65907-30-4 112226-61-6 100784-20-1 69806-34-4 23560-59-0 78587-05-0
CAS 60-51-5
Formula C14H21NO4 C19H17Cl2N3O3 C14H9ClF2N2O2 C5H12NO3PS2 C21H22ClNO4 C19H22N2O3 C7H14N4O3 C8H19O3PS3 C9H10Cl2N2O C10H14N2 C15H33N3O2 C17H13ClFN3O C11H15NO2S C11H15NO2S2 C11H15NO3S C11H19N3O C25H28O3 C17H17N3OS C13H22NO3PS C13H22NO5PS C13H22NO4PS C19H17ClN4 C14H17Cl2NO2 C17H19NO4 C20H33NO C24H27N3O4 C10H15O4PS2 C10H15O5PS2 C12H4Cl2F6N4OS C15H12F3NO4 C13H4Cl2F6N4O4 C12H6F2N2O2 C14H13F4N3O2S C21H11ClF6N2O3 C10H11F3N2O C14H8Cl3F3N2O C21H16ClFN4O5 C7H5Cl2FN2O3 C16H13F2N3O C9H18NO3PS2 C18H26N2O5S C18H19ClN2O2 C13H15ClN6O7S C15H11ClF3NO4 C9H12ClO4P C17H21ClN2O2S
Compound Diethofencarb Difenoconazole Diflubenzuron Dimethoate Dimethomorph Dimoxystrobin Dinotefuran Disulfoton sulfoxide Diuron DMPF Dodine Epoxiconazole Ethiofencarb Ethiofencarb sulfone Ethiofencarb sulfoxide Ethirimol Etofenprox Fenamidone Fenamiphos Fenamiphos sulfone Fenamiphos sulfoxide Fenbuconazole Fenhexamid Fenoxycarb Fenpropimorph Fenpyroximate Fenthion sulfoxide Fenthion sulfone Fipronil* Fluazifop acid* Fluazinam* Fludioxonil* Flufenacet Flufenoxuron Fluometuron Fluopicolide Fluoxastrobin Fluroxypyr* Flutriafol Fosthiazate Furathiocarb Halofenozide Halosulfuron-methyl* Haloxyfop acid* Heptenophos Hexythiazox
0.9975 0.9999 0.9979 0.9990 0.9995 0.9998 0.9999 1.0000 0.9984 0.9979 0.9989 0.9903 0.9989 0.9996 0.9997 0.9954 0.9968 0.9968 0.9994 0.9998 0.9999 0.9949 0.9999 0.9988 0.9996 0.9993 0.9991 0.9979 0.9966 0.9990 0.9967 0.9977 0.9970 0.9967 0.9978 0.9993 0.9994 0.9996 0.9991 0.9963 0.9993 0.9951 0.9980
R 2
Table 1 Studied compounds and their chemical formulas, CAS numbers, SRMs, retention times, limits of detection and R 2
%RSD (10ppb) 5.0 1.0 1.1 4.4 1.5 1.7 1.8 2.3 2.8 3.8 2.4 2.7 2.1 1.5 0.9 7.4 4.6 4.5 2.9 1.6 3.1 9.7 1.9 2.4 1.1 0.7 0.9 2.3 2.3 3.2 1.3 1.0 0.7 9.5 1.6 2.3 1.2 1.3 4.6 4.8 1.9 1.3 9.7
Transition 2 LOD (ppb) 0.56 0.31 1.00 2.36 0.26 0.10 0.13 0.36 0.22 2.03 0.22 0.50 0.30 0.09 0.41 5.43 1.19 2.61 1.79 0.39 0.87 3.23 0.21 0.22 1.00 0.18 0.25 1.00 0.49 0.18 0.13 0.60 0.45 5.76 0.12 0.20 0.05 0.09 3.12 3.53 0.41 0.29 5.00
Transition 1 LOD (ppb) 0.35 0.17 0.50 1.32 0.09 0.05 0.17 0.22 0.14 0.72 0.10 0.50 0.03 0.10 0.12 1.41 0.81 2.24 0.72 0.29 0.13 2.63 0.22 0.13 0.50 0.12 0.09 0.50 0.28 0.20 0.05 0.05 0.08 4.03 0.25 0.10 0.02 0.09 1.76 0.92 0.40 0.09 1.09
(You can find the whole table linked to the shortened one below.)
Pear extract RT (min.) 16.4 3.0 7.2 16.1 13.4 8.5 4.3 3.9 9.3 3.4 11.1 11.8 12.1 9.8 12.7 12.1 12.5 9.9 13.0 15.3 8.4 4.1 3.7 10.3 7.1 11.1 10.2 11.8 14.0 7.2 10.8 13.0 14.7 14.4 6.5 8.3 14.6 14.8 14.2 11.7 13.2 14.0 10.3
Transition 2 891 > 567 184 > 49 223 > 99 559 > 181 270 > 162 208 > 89 240 > 86 207 > 132 370 > 139 231 > 92 216 > 104 318 > 77 404 > 344 224 > 167 382 > 116 453 > 179 343 > 140 276 > 81 376 > 70 312 > 57 208 > 191 223 > 166 207 > 75 202 > 127 192 > 132 222 > 123 236 > 87 482 > 177 361 > 99 222 > 104 213 > 46 395 > 91 360 > 268 303 > 102 250 > 169 190 > 163 409 > 145 346 > 43 357 > 176 437 > 392 320 > 70 336 > 159 219 > 125
Transition 1 891 > 305 184 > 143 223 > 126 559 > 208 270 > 238 208 > 116 240 > 223 207 > 89 370 > 261 231 > 156 216 > 174 318 > 132 404 > 372 224 > 109 382 > 180 453 > 297 343 > 307 274 > 79 376 > 159 312 > 238 208 > 75 223 > 106 207 > 88 202 > 145 192 > 160 222 > 165 236 > 143 482 > 284 361 > 155 222 > 92 213 > 72 395 > 175 360 > 164 303 > 138 250 > 132 190 > 136 409 > 186 346 > 278 357 > 154 435 > 390 318 > 70 336 > 187 219 > 161
71751-41-2 30560-19-1 135410-20-7 101007-06-1 15972-60-8 116-06-3 1646-88-4 1646-87-3 120923-37-7 3337-71-1 1912-24-9 86-50-0 131860-33-8 22781-23-3 177406-68-7 125401-92-5 188425-85-6 1689-84-5 116255-48-2 23184-66-9 34681-10-2 34681-23-7 34681-24-8 63-25-2 10605-21-7 1563-66-2 5234-68-4 500008-45-7 470-90-6 1698-60-8 15545-48-9 143807-66-3 99129-21-2 74115-24-5 210880-92-5 41814-78-2 141517-21-7 68694-11-1 64628-44-0 26644-46-2 131983-72-7 156052-68-5 94-75-7
CAS
global w430×h280 Formula C48H72O14 C4H10NO3PS C10H11ClN4 C26H21F6NO5 C14H20ClNO2 C7H14N2O2S C7H14N2O4S C7H14N2O3S C9H15N5O7S2 C8H10N2O4S C8H14ClN5 C10H12N3O3PS2 C22H17N3O5 C11H13NO4 C18H24FN3O3S Benthiavalicarb-isopropyl C19H17N4NaO8 C18H12Cl2N2O C7H3Br2NO C13H12BrCl2N3O C17H26ClNO2 C7H14N2O2 S C7H14N2O4S C7H14N2O3S Butocarboxim sulfoxide C12H11NO2 C9H9N3O2 C12H15NO3 C12H13NO2S C18H14BrCl2N5O2 C12H14Cl3O4P C10H8ClN3O C10H13ClN2O C24H30N2O3 C17H26ClNO3S C14H8Cl2N4 C6H8ClN5O2S C9H7N3S C20H19F3N2O4 C15H15ClF3N3O C15H10ClF3N2O3 C10H14Cl6N4O2 C17H20ClN3O C14H16Cl3NO2 C8H6Cl2O3 * Negative electrospray ionisation
Food safety Compound Avermectin B1a Acephate Acetamiprid Acrinathrin Alachlor Aldicarb Aldicarb sulfone Aldicarb sulfoxide Amidosulfuron Asulam Atrazine Azinphos -methyl Azoxystrobin Bendiocarb Bispyribac sodium Boscalid Bromoxynil* Bromuconazole Butachlor Butocarboxim Butocarboxim sulfone Carbaryl Carbendazim Carbofuran Carboxin Chlorantraniliprole* Chlorfenvinfos Chloridazon Chlorotoluron Chromafenozide Clethodim Clofentezine Clothianidin Tricyclazole Trifloxystrobin Triflumizole Triflumuron* Triforine Triticonazole Zoxamide 2,4-D* 12