Page 7 - Shimadzu LCMS-9050
P. 7
The charts on the left each plot the mass errors with respect to theoretical values for six antibiotic
components (with molecular weights ranging from 265 to 1203 Da) analyzed continuously under normal
laboratory conditions. When three components of antibiotics were analyzed each in positive and negative
ion mode, the exact masses of all components showed stable mass accuracy within ±2 ppm of the
theoretical values for 72 hours without a single mass calibration. The LCMS-9050's precisely controlled
temperature control system minimizes the effects of changes in room temperature and achieves stable mass
accuracy without the need for calibration. Mass accuracy (external calibration) within ±3 ppm was obtained
for all components in the simultaneous analysis of positive and negative ions. Stable mass accuracy is
maintained even during long-time continuous analysis using the positive-negative switching mode.
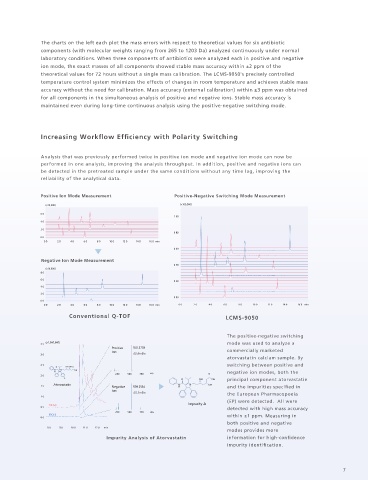
Increasing Workflow Efficiency with Polarity Switching
Analysis that was previously performed twice in positive ion mode and negative ion mode can now be
performed in one analysis, improving the analysis throughput. In addition, positive and negative ions can
be detected in the pretreated sample under the same conditions without any time lag, improving the
reliability of the analytical data.
Positive Ion Mode Measurement Positive-Negative Switching Mode Measurement
(x10,000) (x100,000)
6 0
1 00
4 0
2 0
0 80
0 0
0 0 2 0 4 0 6 0 8 0 10 0 12 0 14 0 16 0 min
0 60
Negative Ion Mode Measurement
0 40
(x10,000)
8 0
6 0 0 20
4 0
2 0
0 00
0 0
0 0 2 0 4 0 6 0 8 0 10 0 12 0 14 0 16 0 min 0 0 2 0 4 0 6 0 8 0 10 0 12 0 14 0 16 0 min
Conventional Q-TOF LCMS-9050
The positive-negative switching
3 5 (x1,000,000) mode was used to analyze a
Positive 541.2703
Ion commercially marketed
3 0 ∆0.6mDa
atorvastatin calcium sample. By
2 5 switching between positive and
O OH OH O
H 250 500 750 m/z O negative ion modes, both the
N N OH
2 0
F O OH OH principal component atorvastatin
Atorvastatin N N OH
1 5 Negative 539.2554 H and the impurities specified in
Ion ∆0.3mDa
1 0 the European Pharmacopoeia
(EP) were detected. All were
TIC (+) Impurity A
detected with high mass accuracy
0 5
250 500 750 m/z
TIC (-) within ±1 ppm. Measuring in
0 0
both positive and negative
8 0 9 0 10 0 11 0 12 0 min
modes provides more
Impurity Analysis of Atorvastatin information for high-confidence
impurity identification.
7