Page 29 - Microorganism Species Analysis
P. 29
LCMS-2020 LCMS-2020
Analysis of Nucleic Acids, Amino Acids, and Aflatoxins
Microorganism Solutions Microorganism Solutions
Data Data
Analysis of Amino Acids Analysis of Compounds Related to Nucleic Acids
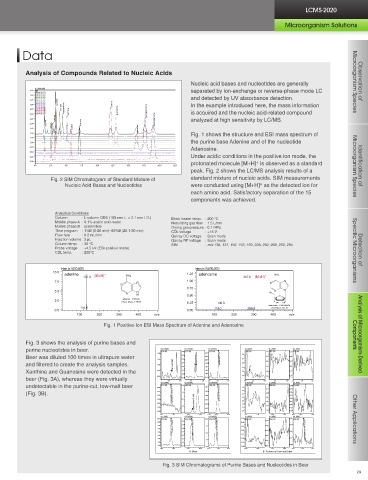
Amino acid is a generic term for compounds that contain an amino group and a carboxyl group. Several hundred of them Nucleic acid bases and nucleotides are generally Microorganism Species Observation of
(x1,000,000)
exist naturally. Amino acids are the basic units comprising proteins, which are one of the major biological components. 3.75 TIC separated by ion-exchange or reverse-phase mode LC
169.00 (4.77)
3.50 269.00 (9.52)
137.00 (1.52)
They provide the materials to synthesize the neurotransmitters and low-molecular-weight bioactive compounds and even 3.25 153.00 (3.21) and detected by UV absorbance detection.
152.00 (1.00)
284.00 (6.36)
136.00 (6.42) guanine
Observation of
268.00 (4.52)
alone offer a variety of bioactivities. They are widely researched in the fields of pharmaceuticals and foods and many amino 3.00 112.00 (1.00) uric acid inosine In the example introduced here, the mass information
113.00 (4.56)
2.75 127.00 (4.52) hypoxanthine guanosine
245.00 (8.16)
acids have been used for health food supplements in recent years. 2.50 252.00 (4.77) xanthine deoxyguanosine is acquired and the nucleic acid-related compound
228.00 (3.91)
Microorganism Species
2.25 adenine deoxyadenosine analyzed at high sensitivity by LC/MS.
Analytical Conditions 2.00 cytosine thymine
( x100,000) Column : Shim-pack FC-ODS 1.75 deoxycytidine uracil uridine adenosine
TIC (150 mm L. × 2.0 mm I.D.) 1.50
3.00 76.00 (7.04) Fig. 1 shows the structure and ESI mass spectrum of
90.00 (4.84) Mobile phase : ion pair reagent-water / acetonitrile
147.00 (1.33) 1.25
2.75 175.00 (1.00) Time program : gradient elution 1.00 the purine base Adenine and of the nucleotide
156.00 (1.43)
134.00 (4.17) Flow rate : 0.2 mL/min
2.50 0.75
148.00 (2.35) arginine Adenosine.
106.00 (4.39) Injection volume : 3 µL
2.25 120.00 (2.20) 0.50
182.00 (1.42) Column temp. : 40 °C Under acidic conditions in the positive ion mode, the
150.00 (1.29) glycine alanine 0.25
2.00 166.00 (1.00) lysine Probe voltage : +4.5 kV (ESI-positive mode)
118.00 (1.07) 0.00 +
132.00 (1.09) histidine CDL temp. : 200 °C protonated molecule [M+H] is observed as a standard
1.75 aspartic acid 0.0 2.5 5.0 7.5 10.0 12.5 15.0 17.5 20.0 22.5 Microorganism Species Identification of
116.00 (1.70)
205.00 (1.00) glutamic acid Block heater temp. : 200 °C
1.50 241.00 (2.34) serine threonine tyrosine phenylalanine peak. Fig. 2 shows the LC/MS analysis results of a
133.00 (12.47) methionine Nebulizing gas flow : 1.5 L/min
1.25 valine Drying gas pressure : 0.1 MPa standard mixture of nucleic acids. SIM measurements
proline isoleucine Fig. 2 SIM Chromatogram of Standard Mixture of
leucine CDL voltage : +10 V
1.00 cystine tryptophan +
Qarray DC voltage : Scan mode Nucleic Acid Bases and Nucleotides were conducted using [M+H] as the detected ion for
0.75
Identification of
Qarray RF voltage : Scan mode
0.50 SIM : m/z 76, 90, 147, 175, 156, 134, 148, each amino acid. Satisfactory separation of the 15
106, 120, 182, 122, 150, 166, 118,
Microorganism Species
0.25 components was achieved.
132, 116, 205, 241
0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 10.0 11.0 12.0 13.0
Analytical Conditions
Column : L-column ODS (150 mm L. × 2.1 mm I.D.) Block heater temp. : 200 °C
Fig. 1 SIM Chromatogram of a Standard Mixture of 18 Amino Acid Components Mobile phase A : 0.1% acetic acid–water Nebulizing gas flow : 1.5 L/min
Mobile phase B : acetonitrile Drying gas pressure : 0.1 MPa
Time program : 1%B (0-20 min)–80%B (20.1-30 min) CDL voltage : +15 V
Flow rate : 0.2 mL/min Qarray DC voltage : Scan mode
Injection volume : 3 µL Qarray RF voltage : Scan mode
( x100,000) Column temp. : 30 °C SIM : m/z 136, 137, 152, 153, 169, 228, 252, 268, 269, 284
3.00 TIC Probe voltage : +4.5 kV (ESI-positive mode)
76.00 (1.00) CDL temp. : 200°C Specific Microorganisms Detection of
2.75 90.00 (1.00)
147.00 (1.00)
175.00 (1.00)
2.50 156.00 (1.00)
134.00 (1.00)
148.00 (1.00)
2.25 106.00 (1.00)
Detection of
120.00 (1.00) Inten.(x1,000,000) Inten.(x10,000,000)
2.00 182.00 (1.00) arginine 10.0
150.00 (1.00) adenine + 1.25 adenosine NH 2
166.00 (1.00) (M+H) NH 2 (M+H) +
1.75 118.00 (1.00) 135. 9 267.9
132.00 (1.00) phenylalanine 7.5 1.00 N
116.00 (1.00) N N
1.50 205.00 (1.00) glutamic acid N
Specific Microorganisms
241.00 (1.00) lysine 0.75
1.25 alanine leucine 5.0 N N
glycine valine
N N 0.50 HO
1.00 H O
aspartic acid histidine isoleucine H H
threonine methionine 2.5 adenine C5H5N5
0.75 serine Exact Mass: 135.05 0.25 136.0 H OH OH H
proline tyrosine adenosine C10H13N5O4
0.50 118. 9 119.0 289.9 Exact Mass: 267.10
0.0 0.00
0.25 100 200 300 400 m/z 100 200 300 400 m/z
0.00
0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 10.0 11.0 12.0 13.0 Fig. 1 Positive-Ion ESI Mass Spectrum of Adenine and Adenosine
Fig. 2 SIM Chromatogram of Amino Acids in Soy Sauce Components Analysis of Microorganism-Derived
Fig. 3 shows the analysis of purine bases and
purine nucleotides in beer. (x10,000) 4.0 152.00 (x10,000) (x1,000) 7.0 152.00 7.75 153.00
(x10,000)
(x1,000)
(x1,000)
Components
136.00
136.00
153.00
1.50 3.5 2.50 7.0
Beer was diluted 100 times in ultrapure water 1.25 3.0 guanine/3.312 2.25 2.00 xanthine/5.133 6.5 6.5 6.0 7.50 7.25
and filtered to create the analysis samples. 1.00 adenine/2.963 2.5 2.0 1.75 1.50 6.0 5.5 7.00
An LC system with a cation exchange column, pre- or post-column derivatization, and fluorescence detection is generally 0.75 1.5 1.25 5.5 5.0 6.75
Xanthine and Guanosine were detected in the 1.0 1.00 5.0
used for the analysis of amino acids. It offers compositional analysis of the protein content of culture fluid and protein 0.50 0.5 0.75 4.5 4.5 6.50
Analysis of Microorganism-Derived
beer (Fig. 3A), whereas they were virtually 2.5 4.9 2.5 5.0 3.2 5.0 7.2 0.9 2.5 4.9 2.5 5.0 3.2 5.0 7.2
structural analysis. However, a dedicated system is required and separation takes a comparatively long time. A more (x10,000) 10.0 268.00 (x100,000) (x10,000) 3.2 (x1,000) 3.10 284.00
(x1,000)
(x10,000)
undetectable in the purine-cut, low-malt beer 4.00 1.50 3.3
137.00
284.00
137.00
268.00
general-purpose, faster system is desirable. 3.75 7.5 1.25 3.2 3.1 3.05 3.00
(Fig. 3B). 3.50 hypoxanthine/4.417 1.00 guanosine/12.754 3.1 3.0 2.95
3.25 5.0 0.75 3.0 2.9 2.9 2.90 2.85
3.00 adenosine/15.848 0.50 2.8 2.8
The above shows an example of amino acid analysis by LC/MS. Electrospray ionization (ESI) is suitable for the analysis of 2.75 2.5 0.25 2.7 2.7 2.80 2.75
amino acids, as amino acids are amphiprotic compounds that have a positive or negative charge according to the pH of the 2.50 2.5 5.0 0.0 15.0 17.5 0.00 11.0 12.5 15.0 2.6 2.5 5.0 2.6 15.0 17.5 2.70 11.0 12.5 15.0
(x1,000) (x10,000) (x10,000) 4.75 (x1,000) (x1,000) 3.50 (x1,000)
aqueous solution. 11.0 10.0 269.00 4.0 252.00 10.0 268.00 4.50 269.00 7.25 252.00 268.00 Other Applications
9.0 inosine/11.929 3.5 7.5 4.25 7.00 3.25
8.0 3.0 deoxyadenosine/18.183 deoxyguanosine/16.950 4.00 deoxyguanosine/17.683
7.0 2.5 5.0 6.75 3.00
Fig. 1 shows the SIM chromatogram of the standard mixture of 18 amino acid components. The separation required for the 6.0 2.0 3.75 6.50
5.0 1.5 2.5 3.50 2.75
quantitation of 18 amino acid components can be completed in approximately 12 minutes. Fig. 2 shows the analysis of the 4.0 1.0 3.25 6.25
Other Applications
3.0 0.5 0.0 3.00 6.00 2.50
amino acids contained in soy sauce (diluted 250 times). 10.0 12.5 17.5 20.0 15.5 17.5 19.5 10.0 12.5 17.5 20.0 15.5 17.5 19.5
A: Beer B: Purine-cut low-malt beer
This type of system is widely used for the analysis of the free amino acids in vinegar or Japanese sake. Fig. 3 SIM Chromatograms of Purine Bases and Nucleotides in Beer
28 29