Page 12 - Guide to Lithium-ion Battery Solutions
P. 12
Thermal Analysis
Evaluate the decomposition characteristics and thermal stability of battery materials during heating Purpose Samples
Determining the melting temperature and Three types of separators
calorimetry of separators
Differential Scanning Calorimeter
DSC-60 Plus
• Examination of Lithium-ion Battery safety Data
• Selection of compound for electrode material and selection of
compounding conditions
• Evaluation of physical properties of polymer materials by crystallinity
In some cases, lithium-ion batteries may generate abnormal heat due to overcharging, which may lead to problems
such as ignition, in the worst case. To ensure the safety of the battery, it is important to evaluate the decomposition
characteristics and thermal stability of each component by DSC during heating.
Purpose Samples
Evaluation of decomposition characteristics Charged/Uncharged electrode active
and thermal stability of each component material and electrolyte solution
Data
DSC Measurement of Separators
Result
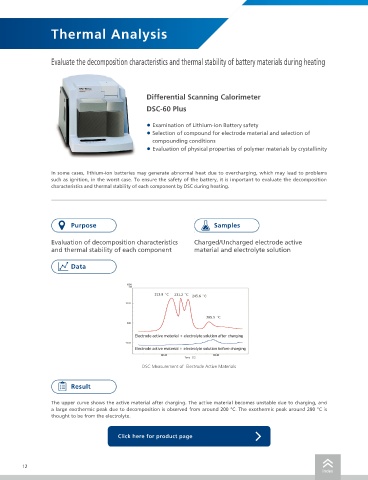
DSC Measurement of Electrode Active Materials The endothermic peaks were observed at around 100 °C to 140 °C, which is expected to be the melting point of
polyethylene. When the separator is exposed to high temperature, it shrinks near the melting temperature, which affects
the insulation. For safety reasons, it is necessary to know the temperature at which the separator contracts, and the
Result measurement of the melting temperature by DSC is an indicator of the contraction temperature.
The upper curve shows the active material after charging. The active material becomes unstable due to charging, and
a large exothermic peak due to decomposition is observed from around 200 °C. The exothermic peak around 290 °C is
thought to be from the electrolyte.
Click here for product page Click here for product page
12 13
index index