Page 85 - Application Handbook - Liquid Chromatography
P. 85
LAAN-A-LC-E216
Application High Performance Liquid Chromatography
News LC/MS/MS Analysis of Impurities in Active Pharmaceutical
Ingredients Using the Co-Sense for Impurities System
No.L440
Detection of impurities in active pharmaceutical Table 1 Analytical Conditions
ingredients (APIs) is often conducted using an HPLC-UV Column (Ⅰ) : Shim-pack VP-ODS (150 mm L. × 4.6 mm I.D., 4.6 µm)
method. However, qualitative and quantitative analysis Mobile Phase : Methanol / 50 mmol/L Phosphate buffer pH7.0 (3/2)
of impurities requires not only the separation of the Flowrate : 1.0 mL/min
impurities from the major component, but also Column Temp. : 30 °C
Injection Volume : 20 µL
separation among impurities themselves. The time and Detection (A) : UV 290 nm
effort required to establish effective analytical
conditions for this type of analysis are significant. MS detection requires analysis to be conducted using a
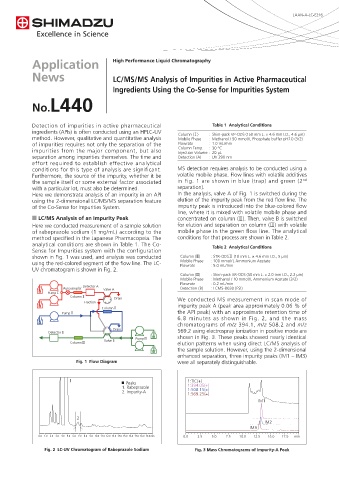
Furthermore, the source of the impurity, whether it be volatile mobile phase. Flow lines with volatile additives
nd
the sample itself or some external factor associated in Fig. 1 are shown in blue (trap) and green (2
with a particular lot, must also be determined. separation).
Here we demonstrate analysis of an impurity in an API In the analysis, valve A of Fig. 1 is switched during the
using the 2-dimensional LC/MS/MS separation feature elution of the impurity peak from the red flow line. The
of the Co-Sense for Impurities System. impurity peak is introduced into the blue-colored flow
line, where it is mixed with volatile mobile phase and
n LC/MS Analysis of an Impurity Peak concentrated on column (Ⅱ). Then, valve B is switched
Here we conducted measurement of a sample solution for elution and separation on column (Ⅲ) with volatile
of rabeprazole sodium (1 mg/mL) according to the mobile phase in the green flow line. The analytical
method specified in the Japanese Pharmacopeia. The conditions for that process are shown in Table 2.
analytical conditions are shown in Table 1. The Co-
Sense for Impurities system with the configuration Table 2 Analytical Conditions
shown in Fig. 1 was used, and analysis was conducted Column (Ⅱ) : STR-ODSⅡ (10 mm L. × 4.6 mm I.D., 5 µm)
using the red-colored segment of the flow line. The LC- Mobile Phase : 100 mmol/L Ammonium Acetate
Flowrate
: 5.0 mL/min
UV chromatogram is shown in Fig. 2.
Column (Ⅲ) : Shim-pack XR-ODS (50 mm L. × 2.0 mm I.D., 2.2 µm)
Mobile Phase : Methanol / 10 mmol/L Ammonium Acetate (3/2)
Flowrate : 0.2 mL/min
Detector A
Autosampler Valve A Detection (B) : LCMS-8030 (ESI)
PumpⅠ
ColumnⅠ
Drain We conducted MS measurement in scan mode of
Fraction
ColumnⅡ impurity peak A (peak area approximately 0.06 % of
PumpⅡ the API peak) with an approximate retention time of
6.8 minutes as shown in Fig. 2, and the mass
chromatograms of m/z 394.1, m/z 508.2 and m/z
Drain
Detector B 569.2 using electrospray ionization in positive mode are
PumpⅢ shown in Fig. 3. These peaks showed nearly identical
Valve B
ColumnⅢ elution patterns when using direct LC/MS analysis of
the sample solution. However, using the 2-dimensional
enhanced separation, three impurity peaks (IM1 – IM3)
Fig. 1 Flow Diagram were all separately distinguishable.
1 Peaks 1:TIC(+)
1. Rabeprazole 1:394.05(+)
1:508.15(+)
2. Impurity-A 1:569.25(+)
IM1
2
IM2
IM3
0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 10.0 11.0 12.0 13.0 14.0 15.0 16.0 17.0 18.0 19.0 min 0.0 2.5 5.0 7.5 10.0 12.5 15.0 17.5 min
Fig. 2 LC-UV Chromatogram of Rabeprazole Sodium Fig. 3 Mass Chromatograms of Impurity-A Peak