Page 7 - Shimadzu Journal vol.2 Issue1
P. 7
Human Health Human Health
2-2 Sample Preparation 3. Results and Discussion being reported in a study of human exposure to BPA through
Quantitative analysis of bisphenol A in water Standard solutions. Stock solutions of BPA and BPA-G were prepared Since BPA is a constituent of many plastics including some used in drinking water. An example of the analysis of BPA in type I
15
in methanol at final concentrations of 1 mg/mL each. Working laboratory plastic ware and cap liners of vials and bottles, the purified laboratory water is shown in Fig. 4. It should be noted
and serum using UHPLC-MS-MS standards were made by serial dilution from stock solutions. solvents and materials used for BPA and BPA-G extraction and that even many laboratory water purification systems produce
Calibration standards were prepared by mixing 1 µL of each working analysis had to be tested for BPA. Several brands of HPLC-grade water contaminated with BPA, since they often contain
standard with 24 µL blank mouse serum or water and vortex mixing. methanol, methyl-tert-butyl ether and acetonitrile tested positive polysulfone filters that leach BPA monomer. 12
1
1
Richard B. van Breemen , Guannan Li , Yang Yuan , and Ke Huang 1 Serum. Each unknown serum sample (25 µL) or calibration standard for BPA. Eventually, all laboratory ware and solvents utilized for
1
1 Department of Medicinal Chemistry and Pharmacognosy, University of Illinois College of Pharmacy, 833 South Wood (25 µL) was mixed with 100 µL acetonitrile containing the surrogate sample preparation and analysis were confirmed to be free of BPA
Street, Chicago, IL 60612 USA standards 5 ng/mL [d6]-BPA and 5 ng/mL [ C 12]-BPA-G. The mixture contamination.
13
was vortexed for 1 min, centrifuged for 15 min at 13000 x g at 4 C, and The recoveries of BPA and BPA-G from serum using
°
ABSTRACT then the supernatant was removed and evaporated to dryness. The solvent/solvent extraction were ʙ90% and ʙ87%, respectively.
residue was reconstituted in 25 µL of 50% aqueous methanol, and a 5 The standard curves for BPA and BPA-G in water and serum were
Endocrine disruptors represent a major toxicological and public health issue, and the xenoestrogen bisphenol A (BPA) has received much
attention due to its high volume of production for plastics and widespread human exposure. The measurement of BPA in biological and µL aliquot was injected onto the UHPLC-MS-MS system for analysis. linear. For example, the standard curves for BPA and BPA-G in
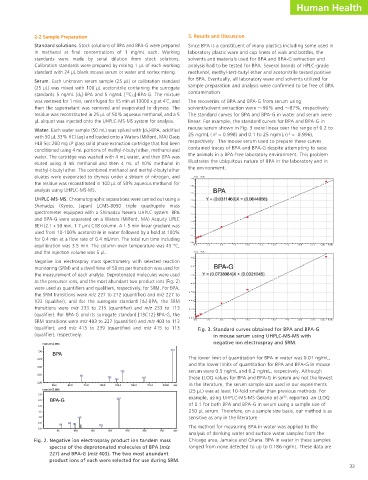
environmental samples is essential for risk assessment. However, such analyses are challenging due to the trace levels of BPA in these Water. Each water sample (50 mL) was spiked with [d6]-BPA, acidified mouse serum shown in Fig. 3 were linear over the range of 0.2 to
2
2
samples and the possibility of false positive determinations due to background contamination. To overcome these limitations, we with 50 µL 33% HCl (aq) and loaded onto a Waters (Milford, MA) Oasis 25 ng/mL ( r = 0.998) and 0.1 to 25 ng/mL (r = 0.996),
developed a highly sensitive and selective method using ultrahigh pressure liquid chromatography (Shimadzu Nexera) and a new HLB 5cc 200 mg LP glass solid phase extraction cartridge that had been respectively. The mouse serum used to prepare these curves
generation triple quadruple mass spectrometer (Shimadzu LCMS-8050). contained traces of BPA and BPA-G despite attempting to raise
conditioned using 4 mL portions of methyl-t-butyl ether, methanol and
water. The cartridge was washed with 4 mL water, and then BPA was the animals in a BPA-free laboratory environment. This problem
Key Words eluted using 4 mL methanol and then 4 mL of 10% methanol in illustrates the ubiquitous nature of BPA in the laboratory and in
Bisphenol A, bisphenol A-glucuronide, serum, UHPLC-MS-MS, LCMS-8050, water quality methyl-t-butyl ether. The combined methanol and methyl-t-butyl ether the environment.
eluates were evaporated to dryness under a stream of nitrogen, and
the residue was reconstituted in 100 µL of 50% aqueous methanol for
1. Introduction
analysis using UHPLC-MS-MS.
4
Used as a plasticizer in epoxy resins such as those lining canned have been reported in neonates. To enable risk assessment of UHPLC-MS-MS. Chromatographic separations were carried out using a
foods, as a constituent of thermal papers used in cash register BPA exposure, BPA and BPA-G need to be determined in serum Shimadzu (Kyoto, Japan) LCMS-8050 triple quadrupole mass
receipts and as a monomer for polycarbonate plastic used in not only of adults but also of neonates and infants, from whom spectrometer equipped with a Shimadzu Nexera UHPLC system. BPA
production of water bottles, bisphenol A (BPA) is a high volume serum sample sizes are typically quite small. 5 and BPA-G were separated on a Waters (Milford, MA) Acquity UPLC
industrial chemical with considerable human and environmental BPA has been measured in surface water samples using gas BEH (2.1 x 50 mm, 1.7 µm) C18 column. A 1.5 min linear gradient was
1,2
exposure. BPA (Fig. 1) has estrogenic effects and has been chromatography-tandem mass spectrometry (GC-MS), high used from 10-100% acetonitrile in water followed by a hold at 100%
6
shown to function as an endocrine disruptor. There is concern performance liquid chromatography-tandem mass spectrometry for 0.4 min at a flow rate of 0.4 mL/min. The total run time including
3
that environmental BPA exposure can adversely impact aquatic life (HPLC-MS-MS) and ultrahigh pressure liquid equilibration was 3.5 min. The column oven temperature was 45 °C,
7
and human health, especially in children who might be more chromatography-MS-MS (UHPLC-MS-MS). Most methods for the and the injection volume was 5 µL.
8
sensitive to endocrine disruptors.
measurement of BPA and BPA-G involve chemical or enzymatic Negative ion electrospray mass spectrometry with selected reaction
deconjugation to convert BPA-G to BPA prior to quantitative monitoring (SRM) and a dwell time of 50 ms per transition was used for
9
analysis. For example, Kosarac, et al. enzymatically deconjugated the measurement of each analyte. Deprotonated molecules were used
BPA-G prior to derivatization and measurement of total BPA in as the precursor ions, and the most abundant two product ions (Fig. 2)
serum using GC-MS-MS. Among the few methods that measure were used as quantifiers and qualifiers, respectively, for SRM. For BPA,
both BPA and BPA-G directly in serum, most rely on the SRM transitions were m/z 227 to 212 (quantifier) and m/z 227 to
10
HPLC-MS-MS or UHPLC-MS-MS and utilize negative ion 133 (qualifier); and for the surrogate standard [d6]-BPA, the SRM
11
electrospray without need for derivatization. transitions were m/z 233 to 215 (quantifier) and m/z 233 to 113
We developed methods based on UHPLC-MS-MS for the analysis (qualifier). For BPA-G and its surrogate standard [13C12]-BPA-G, the
of BPA in water and analysis of both BPA and intact BPA-G in SRM transitions were m/z 403 to 227 (quantifier) and m/z 403 to 113
serum. Unlike GC-MS-MS assays, our approach requires no (qualifier); and m/z 415 to 239 (quantifier) and m/z 415 to 113 Fig. 3. Standard curves obtained for BPA and BPA-G
Fig. 1. Chemical structures of BPA and its primary derivatization of BPA, and unlike GC-MS-MS and some (qualifier), respectively. in mouse serum using UHPLC-MS-MS with
human metabolite, BPA-glucuronide. HPLC-MS-MS based assays, our method avoids hydrolysis of negative ion electrospray and SRM.
BPA-G. UHPLC was utilized instead of HPLC to shorten
Human exposure to BPA can result from such diverse sources as chromatographic separation time (less than 2 min per analysis), The lower limit of quantitation for BPA in water was 0.01 ng/mL,
consumption of canned foods, drinking water, dental composites, and when volumes of serum samples are small, our method is and the lower limits of quantitation for BPA and BPA-G in mouse
and cash register receipts. The large quantity of BPA produced unique in that only 25 µL of serum are required. serum were 0.5 ng/mL and 0.2 ng/mL, respectively. Although
world-wide each year has resulted in BPA contamination of rivers these LLOQ values for BPA and BPA-G in serum are not the lowest
and lakes that can affect wildlife as well as humans using these in the literature, the serum sample size used in our experiments
resources for drinking water. Therefore, methods are needed for 2. Experimental (25 µL) was at least 10-fold smaller than previous methods. For
the quantitative analysis of BPA in water. example, using UHPLC-MS-MS Gerano et al . reported an LLOQ
13
After absorption, BPA is metabolized in the liver of adults to form 2-1 Materials of 0.1 for both BPA and BPA-G in serum using a sample size of
primarily BPA-monoglucuronide (BPA-G; Fig. 1) and to a lesser HPLC-grade solvents (acetonitrile, methanol and water) were 250 µL serum. Therefore, on a sample size basis, our method is as
4
extent, BPA-sulfate, before being excreted in urine. Since the purchased from Burdick & Jackson (Honeywell, Muskegon, MI). sensitive as any in the literature.
13
activities of glucuronyltransferases that conjugate BPA with BPA and [rings- C 12]-BPA-G were purchased from Sigma-Aldrich The method for measuring BPA in water was applied to the
glucuronic acid are low at birth and increase for the next several (St. Louis, MO). Bisphenol A mono-β-D-glucuronide (BPA-G) was analysis of drinking water and surface water samples from the
weeks, neonates might be exposed to higher concentrations of obtained from the Midwest Research Institute (Kansas City, MO), Fig. 2. Negative ion electrospray product ion tandem mass Chicago area, Jamaica and Ghana. BPA in water in these samples
unconjugated BPA than adults. While levels of unconjugated BPA and [d 6]-BPA was purchased from Cambridge Isotope Laboratories spectra of the deprotonated molecules of BPA (m/z ranged from none detected to up to 0.186 ng/mL. These data are
in adult human serum are typically low (≤0.5 ng/mL), higher levels (Andover, MA). 227) and BPA-G (m/z 403). The two most abundant
product ions of each were selected for use during SRM.
32 33