Page 4 - Shimadzu Prominence Nano
P. 4
Performance
High-precision proteome analysis by Prominence nano
Data reliability is realized by high basic performance – Exemplary retention time repeatability –
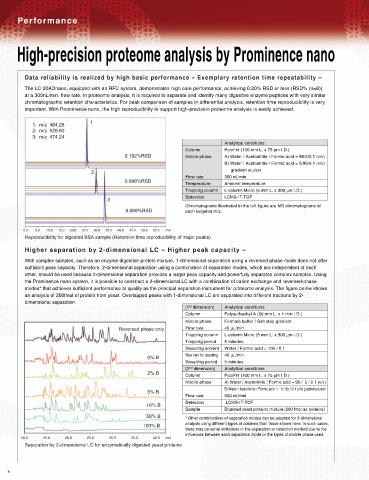
The LC-20ADnano, equipped with an RFC system, demonstrates high core performance, achieving 0.20% RSD or less (RSD% (n=6))
at a 300nL/min. flow rate. In proteome analysis, it is required to separate and identify many digestive enzyme peptides with very similar
chromatographic retention characteristics. For peak comparison of samples in differential analysis, retention time reproducibility is very
important. With Prominence nano, the high reproducibility to support high-precision proteome analysis is easily achieved.
1
1: m/z 464.26
2: m/z 526.60
3: m/z 474.24
Analytical conditions
Column PicoFrit (100 mm L. x 75 μm I.D.)
0.192%RSD Mobile phase A) Water / Acetonitrile / Formic acid = 98/2/0.1 (v/v)
B) Water / Acetonitrile / Formic acid = 5/95/0.1 (v/v)
2 gradient elution
Flow rate 300 nL/min
0.088%RSD
Temperature Ambient temperature
Trapping column L-column Micro (5 mm L. x 300 μm I.D.)
Detection LCMS-IT-TOF
3
Chromatograms illustrated in the left figure are MS chromatograms of
0.066%RSD each targeted m/z.
0.0 5.0 10.0 15.0 20.0 25.0 30.0 35.0 40.0 45.0 50.0 55.0 min
Reproducibility for digested BSA sample (Retention time reproducibility of major peaks)
Higher separation by 2-dimensional LC – Higher peak capacity –
With complex samples, such as an enzyme digestion protein mixture, 1-dimensional separation using a reversed-phase mode does not offer
sufficient peak capacity. Therefore, 2-dimensional separation using a combination of separation modes, which are independent of each
other, should be used because 2-dimensional separation provides a larger peak capacity and powerfully separates complex samples. Using
the Prominence nano system, it is possible to construct a 2-dimensional LC with a combination of cation exchange and reversed-phase
modes* that achieves sufficient performance to quality as the principal separation instrument for proteome analysis. The figure below shows
an analysis of 200fmol of protein from yeast. Overlapped peaks with 1-dimensional LC are separated into different fractions by 2-
dimensional separation.
st
[1 dimension] Analytical conditions
Column Polysulfoethyl A (50 mm L. x 1 mm I.D.)
Mobile phase Formate buffer / Salt step gradient
Reversed phase only Flow rate 40 μL/min
Trapping column L-column Micro (5 mm L. x 300 μm I.D.)
Trapping period 5 minutes
Desalting solvent Water / Formic acid = 100 / 0.1
Flow rate for desalting 40 μL/min
0% B
Desalting period 5 minutes
[2 dimension] Analytical conditions
nd
2% B
Column PicoFrit (100 mm L. x 75 μm I.D.)
Mobile phase A) Water / Acetonitrile / Formic acid = 95 / 5 / 0.1 (v/v)
B) Water / Acetonitrile / Formic acid = 5 / 95 / 0.1 (v/v) gradient elution
5% B
Flow rate 600 nL/min
Detection LCMS-IT-TOF
10% B
Sample Digested yeast proteins mixture (200 fmol as proteins)
50% B * Other combinations of separation modes can be adapted for 2-dimensional
100% B analysis using different types of columns than those shown here. In such cases,
there may be some limitations in the separation or detection method due to the
influences between each separation mode or the types of mobile phase used.
10.0 15.0 20.0 25.0 30.0 35.0 40.0 min
Separation by 2-dimensional LC for enzymatically digested yeast proteins
4