Page 7 - Shimadzu MALDI-8030
P. 7
SOLUTIONS FOR ALL YOUR APPLICATIONS STACKED WITH TECHNOLOGY
Regulatory & Manufacturing Labs Designed for Performance...
DUAL-POLARITY VERSATILITY...for manufacturing/GMP ...Sensitivity and Resolution
Dual-polarity: flexibility of analytes for intact mass analysis
Integrated data security (facilitates 21CFR part 11 compliance): admin-controlled user logins and user traceability
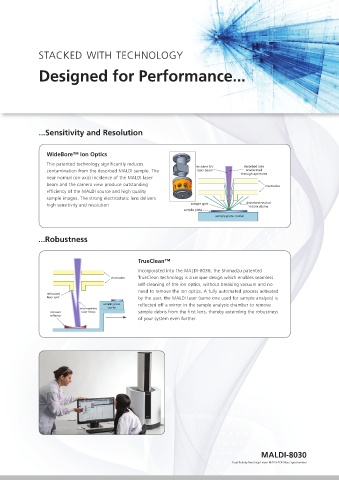
WideBore™ Ion Optics
Save methods: ideal for locked-down GMP protocols following method development and validation
This patented technology significantly reduces
Wide mass range of analysis (up to m/z 500,000) for high mass drug conjugates incident UV desorbed ions
contamination from the desorbed MALDI sample. The laser beam accelerated
through apertures
Automated lockmass feature: suitable for reproducible batch analysis of biosimilars near normal (on-axis) incidence of the MALDI laser
beam and the camera view produce outstanding
Smart choices for (bio-)pharma, polymers, oligonucleotides, genetic testing, quality control. electrodes
efficiency of the MALDI source and high quality
sample images. The strong electrostatic lens delivers
high sensitivity and resolution. sample spot desorbed neutral
Peptide QC in Biopharma sample plate matrix plume
sample plate carrier
2374.959 Class-leading mass resolution
100
90 100 2374.959{r4731}
80 Positive mode 80
70 - intact species not detected %Intensity 60
% Intensity 60 40 20 ...Robustness
50
40
30 -(2 x SO 3 ) 2535.0 0 2370 2375 m/z 2380 2385 100 2452.958{r5963}
20 Intact species %Intensity 80 60
10 not detected 40 TrueClean™
0 20
1600 1800 2000 2200 2400 2600 100 0
m/z 90 2450 2455 2460 Incorporated into the MALDI-8030, the Shimadzu patented
m/z
Negative mode 100
80
An advantage of negative mode analysis can be seen 70 - partial loss of sulfo groups 2452.958 80 60 2532.911{r5938} electrodes TrueClean technology is a unique design which enables seamless
2532.911
during QC of labile peptides from a manufacturing % Intensity 60 - intact peptide detected -SO 3 %Intensity 40 self-cleaning of the ion optics, without breaking vacuum and no
50
^*
batch . In this case the sulfated species are not 40 -SO 3 20 0 need to remove the ion optics. A fully automated process activated
30 2530 2535 2540 2545 refocused
m/z
detected in positive ion mode (above) due to the 20 100 laser spot by the user, the MALDI laser (same one used for sample analysis) is
10 2373.031 80 2373.031{r5955}
preferential ionization, but are clearly seen in 0 60 sample plate reflected off a mirror in the sample analysis chamber to remove
1600 1800 2000 2200 2400 2600 %Intensity 40 intermediate carrier
negative ion mode (right) where the intact peptide m/z 20 concave laser focus sample debris from the first lens, thereby extending the robustness
reflector
and all sulfated species are detected. 0 2370 2375 2380 of your system even further.
m/z
*may require QC Reporter™ software
^ Samples and data kindly obtained by permission from Bachem (UK) Ltd.
Clinical Sample Profiling
The Shimadzu MALDI-8030 is the sensible choice, offering fast sample introduction with a quick MALDI laser
and sample stage thereby providing a high-throughput for your time-to-result cycle.
The integrated barcode reader allows seamless
tracking to reduce misidentification errors when Expert Opinion
*
used with the disposable FlexiMass slides which
have been designed for biological use. “ The new mass spectrometer was really ”
easy to work with. It was perfect for our
*requires SampleStation™ and AuraSolution™ software lipid application in melanoma screening.
Dr Egoitz Astigarraga
IMG Pharma Biotech, Spain
MALDI-8030
Dual-Polarity Benchtop Linear MALDI-TOF Mass Spectrometer