Page 5 - Shimadzu i-Series Method Transfer System
P. 5
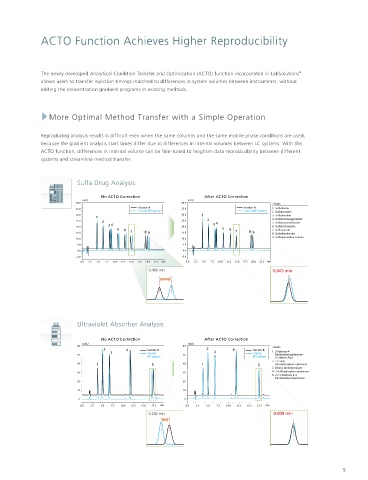
ACTO Function Achieves Higher Reproducibility
™
The newly developed Analytical Condition Transfer and Optimization (ACTO) function incorporated in LabSolutions
allows users to transfer injection timings matched to differences in system volumes between instruments, without
editing the concentration gradient programs in existing methods.
More Optimal Method Transfer with a Simple Operation
Reproducing analysis results is difficult even when the same columns and the same mobile phase conditions are used,
because the gradient analysis start times differ due to differences in internal volumes between LC systems. With the
ACTO function, differences in internal volume can be fine-tuned to heighten data reproducibility between different
systems and streamline method transfer.
Sulfa Drug Analysis
No ACTO Correction After ACTO Correction
mAU mAU
40.0 40.0 Peaks
35.0 Vendor A 35.0 Vendor A 1. Sulfadiazine
i-Series MT system i-Series MT system 2. Sulfamerazine
30.0 30.0 1 3. Sulfadimidine
1
25.0 2 25.0 2 3 4 4. Sulfamethoxypyridazine
5. Sulfamonomethoxine
20.0 3 4 20.0 6. Sulfamethoxazole
5 6 5 6 7 7. Sulfisoxazole
15.0 7 8 9 15.0 8 9 8. Sulfadimethoxine
10.0 10.0 9. Sulfaquinoxaline Sodium
5.0 5.0
0.0 0.0
−5.0 −5.0
0.0 2.5 5.0 7.5 10.0 12.5 15.0 17.5 20.0 22.5 min 0.0 2.5 5.0 7.5 10.0 12.5 15.0 17.5 20.0 22.5 min
0.486 min 0.043 min
Ultraviolet Absorber Analysis
No ACTO Correction After ACTO Correction
mAU mAU
60 60 Peaks
2 4 Vendor B 2 4 Vendor B
3 i-Series 3 i-Series 1. 2-Hydroxy-4-
50 50 Methoxybenzophenone-
MT system MT system 5-Sulfonic Acid
2. 2,2’,4,4’-
40 1 5 40 1 5 Tetrahydroxybenzophenone
3. Ethyl p-Aminobenzoate
30 30 4. 2,4-Dihydroxybenzophenone
5. 2,2’-Dihydroxy-4,4’-
Dimethoxybenzophenone
20 20
10 10
0 0
0.0 2.5 5.0 7.5 10.0 12.5 15.0 17.5 min 0.0 2.5 5.0 7.5 10.0 12.5 15.0 17.5 min
0.252 min 0.009 min
5