Page 4 - Shimadzu ICPE-9800 Series
P. 4
Medical/ High sensitivity
Automatic correction for spectral interference
Pharmaceutical No need for oxygen when introducing organic
solvents
The ICH Q3D guideline established, which aims at harmonization must meet the stringent permitted daily exposure (PDE) values.
among the regulatory authorities in Japan, the U.S., and the EU Validation is also important to guarantee the reliability of analysis
concerning the analysis of elemental impurities in drugs. The values. Moreover, measuring organic solvents, such as DMF, that are
regulations by individual authorities are accordingly revised. As part commonly used for the dissolution of samples requires simplicity and
of the quality control measures for pharmaceuticals, detection limits stability.
With the ICPE-9800 Series
• Highly sensitive, large, 1-inch CCD detector satis es strict detection limit requirements.
In addition to being highly sensitive, the instrument always acquires data for all wavelengths. This makes it easy to quickly con rm the
effect of matrix interference when analyzing tablets or capsules with matrices such as titanium oxide.
• The torch is designed to resist the adhesion of carbon, thereby allowing the direct analysis of organic solvent samples without introducing
oxygen. This realizes stable analysis without the expenditure of extra costs or time.
• Connect to LabSolutions ™ DB/CS using the Multi-Data Register Connection Kit for ICPE (option).
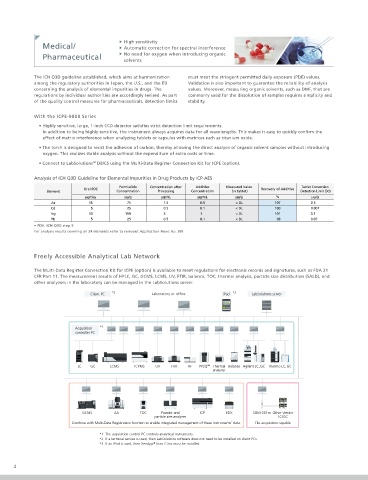
Analysis of ICH Q3D Guideline for Elemental Impurities in Drug Products by ICP-AES
Permissible Concentration after Additive Measured Value Tablet Conversion
Oral PDE Recovery of Additive
Element Concentration Processing Concentration (in tablet) Detection Limit (3σ)
µg/day µg/g µg/mL µg/mL µg/g % µg/g
As 15 75 1.5 0.5 < DL 107 0.5
Cd 5 25 0.5 0.1 < DL 100 0.007
Hg 30 150 3 1 < DL 101 0.1
Pb 5 25 0.5 0.1 < DL 98 0.07
• PDE: ICH Q3D step 5
For analysis results covering all 24 elements refer to relevant Application News No. J99
Freely Accessible Analytical Lab Network
The Multi-Data Register Connection Kit for ICPE (option) is available to meet regulations for electronic records and signatures, such as FDA 21
CFR Part 11. The measurement results of HPLC, GC, GCMS, LCMS, UV, FTIR, balance, TOC, thermal analysis, particle size distribution (SALD), and
other analyzers in the laboratory can be managed in the LabSolutions server.
Client PC *2 Laboratory or of ce iPad *3 LabSolutions server
Acquisition *1
controller PC
LC GC LCMS ICPMS UV FTIR RF PPSQ ™ Thermal Balance Agilent LC, GC Thermo LC, GC
analyzer
GCMS AA TOC Powder and ICP EDX CBM-201m Other Vender
particle size analyzer LC/GC
Combine with Multi-Data Registration function to enable integrated management of these instruments’ data. File acquisition capable
*1 The acquisition contro l PC controls analytical instruments.
*2 If a terminal service is used, then LabSolutions software does not need to be installed on client PCs.
*3 If an iPad is used, then XenApp ® from Citrix must be installed.
4