Page 4 - Application Notebook - Hand Sanitizer Analysis
P. 4
Application Gas Chromatography
News
Analysis of Volatile Impurities in Anhydrous Ethanol
and Ethanol for Disinfection in Accordance with the
No. G331 Purity Test set by the Pharmacopoeias (JP, USP, EP)
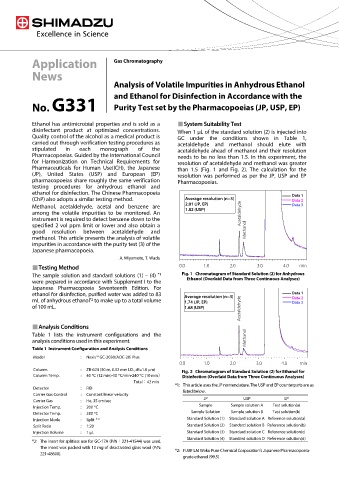
Ethanol has antimicrobial properties and is sold as a System Suitability Test
disinfectant product at optimized concentrations. When 1 μL of the standard solution (2) is injected into
Quality control of the alcohol as a medical product is GC under the conditions shown in Table 1,
carried out through verification testing procedures as acetaldehyde and methanol should elute with
stipulated in each monograph of the acetaldehyde ahead of methanol and their resolution
Pharmacopoeias. Guided by the International Council needs to be no less than 1.5. In this experiment, the
for Harmonization on Technical Requirements for resolution of acetaldehyde and methanol was greater
Pharmaceuticals for Human Use(ICH), the Japanese than 1.5 (Fig. 1 and Fig. 2). The calculation for the
(JP), United States (USP) and European (EP) resolution was performed as per the JP, USP and EP
pharmacopoeias share roughly the same verification Pharmacopoeias.
testing procedures for anhydrous ethanol and
ethanol for disinfection. The Chinese Pharmacopoeia データ1
Data 1
分離度の平均値(n=3))
(ChP) also adopts a similar testing method. Average resolution (n=3 データ2
Data 2
2.01(JP、EP)
Data 3
Methanol, acetaldehyde, acetal and benzene are 2.01 (JP, EP) データ3
1.82(USP)
1.82 (USP)
among the volatile impurities to be monitored. An Acetaldehyde
instrument is required to detect benzene down to the
specified 2 vol ppm limit or lower and also obtain a
good resolution between acetaldehyde and Methanol
methanol. This article presents the analysis of volatile
impurities in accordance with the purity test (3) of the
Japanese pharmacopoeia.
A. Miyamoto, T. Wada
Testing Method 0.0 1.0 2.0 3.0 4.0 min
The sample solution and standard solutions (1) – (4) *1 Fig. 1 Chromatogram of Standard Solution (2) for Anhydrous
were prepared in accordance with Supplement I to the Ethanol (Overlaid Data from Three Continuous Analyses)
Japanese Pharmacopoeia Seventeenth Edition. For
データ1
ethanol for disinfection, purified water was added to 83 Average resolution (n=3) Data 1
分離度の平均値(n=3)
Data 2
mL of anhydrous ethanol to make up to a total volume 1.74 (JP, EP) データ2
*2
1.74(JP 、EP)
データ3
Data 3
of 100 mL. 1.68 (USP) Acetaldehyde
1.68(USP)
Analysis Conditions
Table 1 lists the instrument configurations and the
analysis conditions used in this experiment. Methanol
Table 1 Instrument Configuration and Analysis Conditions
Model : Nexis™ GC-2030/AOC-20i Plus
0.0 1.0 2.0 3.0 4.0 min
Column : ZB-624 (30 m, 0.32 mm I.D., df=1.8 µm)
Fig. 2 Chromatogram of Standard Solution (2) for Ethanol for
Column Temp. : 40 °C (12 min)-10 °C/min-240 °C (10 min) Disinfection (Overlaid Data from Three Continuous Analyses)
Total:42 min
*1: This article uses the JP nomenclature. The USP and EP counterparts are as
Detector : FID
listed below.
Carrier Gas Control : Constant linear velocity
JP USP EP
Carrier Gas : He, 35 cm/sec
Sample Sample solution A Test solution(a)
Injection Temp. : 200 °C
Detector Temp. : 280 °C Sample Solution Sample solution B Test solution(b)
Injection Mode : Split *2 Standard Solution (1) Standard solution A Reference solution(a)
Split Ratio : 1:20 Standard Solution (2) Standard solution B Reference solution(b)
Injection Volume : 1 µL Standard Solution (3) Standard solution C Reference solution(c)
Standard Solution (4) Standard solution D Reference solution(d)
*2: The insert for splitless use for GC-17A (P/N:221-41544) was used.
The insert was packed with 10 mg of deactivated glass wool (P/N:
*2: FUJIFILM Wako Pure Chemical Corporation’s Japanese Pharmacopoeia-
221-48600).
grade ethanol (99.5)