Page 11 - Application Handbook - Liquid Chromatography
P. 11
Application No.L452A
News
n Analysis of Glycans in Antibody Drugs Table 2 Analytical Conditions
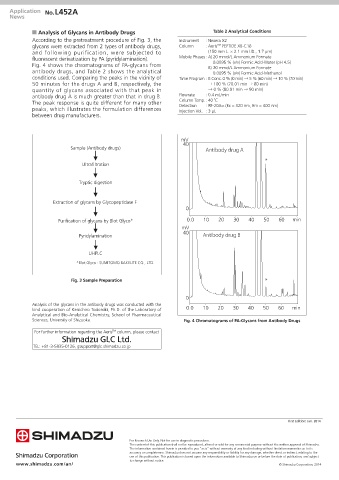
According to the pretreatment procedure of Fig. 3, the Instrument : Nexera X2
glycans were extracted from 2 types of antibody drugs, Column : Aeris PEPTIDE XB-C18
TM
and following purification, were subjected to (150 mm L. × 2.1 mm I.D., 1.7 µm)
fluorescent derivatization by PA (pyridylamination). Mobile Phases : A) 20 mmol/L Ammonium Formate
Fig. 4 shows the chromatograms of PA-glycans from 0.0095 % (v/v) Formic Acid-Water (pH 4.5)
B) 20 mmol/L Ammonium Formate
antibody drugs, and Table 2 shows the analytical 0.0095 % (v/v) Formic Acid-Methanol
conditions used. Comparing the peaks in the vicinity of Time Program : B Conc. 0 % (0 min) → 5 % (60 min) → 10 % (70 min)
50 minutes for the drugs A and B, respectively, the → 100 % (70.01 min → 80 min)
quantity of glycans associated with that peak in → 0 % (80.01 min → 90 min)
antibody drug A is much greater than that in drug B. Flowrate : 0.4 mL/min
The peak response is quite different for many other Column Temp. : 40 ˚C
peaks, which illustrates the formulation differences Detection : RF-20AXS (Ex = 320 nm, Em = 400 nm)
Injection Vol. : 3 µL
between drug manufacturers.
mV
40
Sample (Antibody drugs) "OUJCPEZ ESVH "
Ultra ltration
Tryptic digestion
Extraction of glycans by Glycopeptidase F
0
Puri cation of glycans by Blot Glyco ˎ 0.0 10 20 30 40 50 60 min
mV
40
Pyridylamination "OUJCPEZ ESVH #
UHPLC
ˎ Blot Glyco : SUMITOMO BAKELITE CO., LTD.
Fig. 3 Sample Preparation
0
Analysis of the glycans in the antibody drugs was conducted with the
kind cooperation of Kenichiro Todoroki, Ph.D. of the Laboratory of 0.0 10 20 30 40 50 60 min
Analytical and Bio-Analytical Chemistry, School of Pharmaceutical
Sciences, University of Shizuoka. Fig. 4 Chromatograms of PA-Glycans from Antibody Drugs
TM
For further information regarding the Aeris column, please contact
TEL: +81-3-5835-0126, gsupport@glc.shimadzu.co.jp
First Edition: Jan. 2014
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2014