Page 13 - Pharmaceutical-Shimadzu Total Solution PICS & FDA Compliance
P. 13
Full Support for Regulatory Compliance Total Support for Regulatory Compliance
− Shimadzu's commitment−
The FDA does not, and cannot, certify the hardware, software or services of the customer is extremely important for regulatory compliance. Support for Document Creation
specific manufacturers as regulatory compliant. Therefore, Shimadzu offers the user meticulous total support over the entire
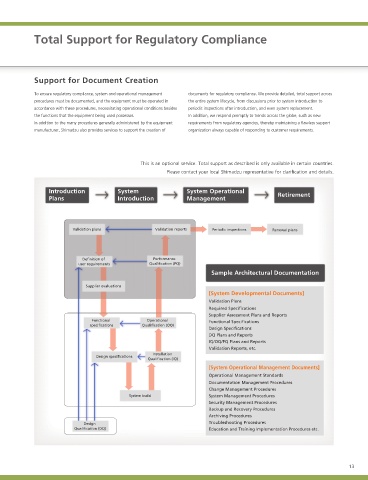
The reason being that compliance with the regulations requires management lifecycle of the product, from consultations before installing a new system to To ensure regulatory compliance, system and operational management documents for regulatory compliance. We provide detailed, total support across
and operating procedures and the associated documentation for the system, regular post-installation inspections. Shimadzu in-house systems remain alert to procedures must be documented, and the equipment must be operated in the entire system lifecycle, from discussions prior to system introduction to
which involves operational requirements additional to the functions offered by the new requirements of regulatory agencies and national and international accordance with these procedures, necessitating operational conditions besides periodic inspections after introduction, and even system replacement.
the product. trends to continue to offer comprehensive support for customer requirements. the functions that the equipment being used possesses. In addition, we respond promptly to trends across the globe, such as new
Consequently, the creation of the company policy and validation master plan by In addition to the many procedures generally administered by the equipment requirements from regulatory agencies, thereby maintaining a flawless support
manufacturer, Shimadzu also provides services to support the creation of organization always capable of responding to customer requirements.
Extensive Shimadzu worldwide customer support network for FDA compliance This is an optional service. Total support as described is only available in certain countries.
This is an optional service. Total support as described is only available in certain countries.
This is an optional service. Total support as described is only available in certain countries.
This is an optional service. Total support as described is only available in certain countries.
This is an optional service. Total support as described is only available in certain countries.
This is an optional service. Total support as described is only available in certain countries.
Please contact your local Shimadzu representative for clarification and details.
Please contact your local Shimadzu representative for clarification and details.
Please contact your local Shimadzu representative for clarification and details.
Please contact your local Shimadzu representative for clarification and details.
Please contact your local Shimadzu representative for clarification and details.
Organizational diagram of 21 CFR regulatory compliance
Overseas Introduction System System Operational
Provision of the Plans Introduction Management Retirement
latest information
Customer
Reaction to FDA Regulation Consultant
requests (Contracted by Shimadzu)
Local Sales Requests
Japan and ·Periodic seminar lecturer Validation plans Validation reports Periodic inspections Renewal plans
International Shimadzu US ·Mock inspections
Marketing Various requests ·Products evaluation
Division (including evaluation and Marketing Center
provision of information)
Regulatory Compliance ·FDA regulation information Definition of Performance
Project Team manager user requirements Qualification (PQ)
·Marketing manager
US FDA Sample Architectural Documentation
·Business Unit Information provision Requests for
·R&D Dept. Technical guidance cooperation including ·FDA regulations Supplier evaluations
·Quality Assurance Dept. participation in seminars ·Provision of regulatory information
·Customer Support Reaction to requests ·Seminars within the FDA [System Developmental Documents]
from FDA including ·Seminars outside of the FDA Validation Plans
seminar participation
Required Specifications
Supplier Assessment Plans and Reports
Functional Operational Functional Specifications
specifications Qualification (OQ)
Design Specifications
DQ Plans and Reports
IQ/OQ/PQ Plans and Reports
Validation Reports, etc.
Installation
Design specifications
Qualification (IQ)
[System Operational Management Documents]
Operational Management Standards
Documentation Management Procedures
Change Management Procedures
System build System Management Procedures
Security Management Procedures
Backup and Recovery Procedures
Archiving Procedures
Design Troubleshooting Procedures
Qualification (DQ) Education and Training Implementation Procedures etc.
12 13