Page 11 - Shimadzu Technologies for COVID-19 Crisis
P. 11
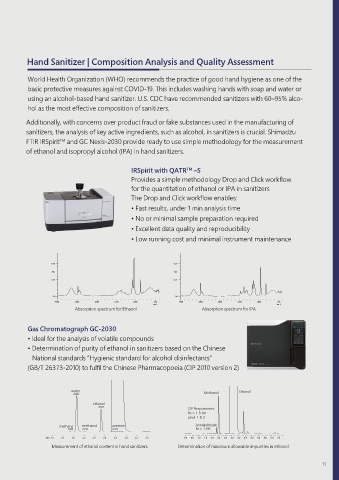
Hand Sanitizer | Composition Analysis and Quality Assessment
World Health Organization (WHO) recommends the practice of good hand hygiene as one of the
basic protective measures against COVID-19. This includes washing hands with soap and water or
using an alcohol-based hand sanitizer. U.S. CDC have recommended sanitizers with 60–95% alco-
hol as the most effective composition of sanitizers.
Additionally, with concerns over product fraud or fake substances used in the manufacturing of
sanitizers, the analysis of key active ingredients, such as alcohol, in sanitizers is crucial. Shimadzu
FTIR IRSpirit and GC Nexis-2030 provide ready to use simple methodology for the measurement
TM
of ethanol and isopropyl alcohol (IPA) in hand sanitizers.
IRSpirit with QATR –S
TM
Provides a simple methodology Drop and Click workflow
for the quantitation of ethanol or IPA in sanitizers
The Drop and Click workflow enables:
• Fast results, under 1 min analysis time
• No or minimal sample preparation required
• Excellent data quality and reproducibility
• Low running cost and minimal instrument maintenance
0.50 0.50
Abs Abs
0.25 0.25
0.0 0.0
4000 3000 2000 1500 1000 500 4000 3000 2000 1500 1000 500
cm-1 cm-1
Absorption spectrum for Ethanol Absorption spectrum for IPA
Gas Chromatograph GC-2030
• Ideal for the analysis of volatile compounds
• Determination of purity of ethanol in sanitizers based on the Chinese
National standards “Hygienic standard for alcohol disinfectants”
(GB/T 26373-2010) to fulfil the Chinese Pharmacopoeia (CIP 2010 version 2)
water Ethanol
2.063 Methanol
ethanol
2.527
CIP Requirement:
Rs > 1.5 for
peak 1 & 2
methane methanol acetone Acetaldehyde
1.935 2.215 2.723 Rs = 1.66
min. 1.6 1.8 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3.4 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 7.5 8.0 8.5 9.0 9.5 min
Measurement of ethanol content in hand sanitizers Determination of maximum allowable impurities in ethanol
11