Page 5 - Shimadzu Nexera Mikros
P. 5
I m p r o v e d De t e c t i o n L i m i t s w i t h M i c r o f l o w I n c o m p ar ab l e M ic r o f l o w R u g g e d n e s s
Monoclonal Antibody Bioanalysis Realize the Benefits of Microflow While Enjoying the
—Nexera Mikros and nSMOL Antibody BA Kit— Ruggedness and Reliability of HPLC
™
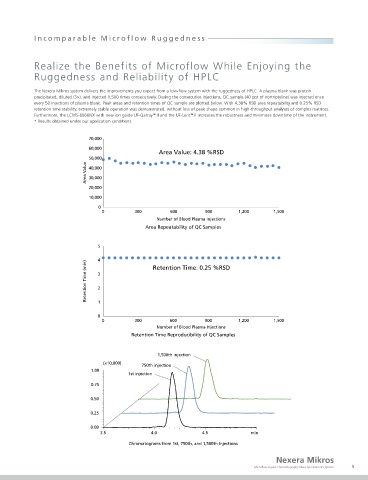
nSMOL (nano-surface and molecular orientation limited proteolysis) is a proprietary, ground-breaking process that enables selective proteolysis of the Fab region of The Nexera Mikros system delivers the improvements you expect from a low-flow system with the ruggedness of HPLC. A plasma blank was protein
antibodies using trypsin-immobilized nanoparticles. Fab-derived peptides are quantified via MRM measurement on a high-sensitivity mass spectrometer. The Nexera precipitated, diluted (3×), and injected 1,500 times consecutively. During the consecutive injections, QC sample (40 ppt of nortriptyline) was injected once
Mikros system achieved signal intensities 12 times higher than with conventional flow rates, with a corresponding lower limit of detection of 0.025 µg/mL and good every 50 injections of plasma blank. Peak areas and retention times of QC sample are plotted below. With 4.38% RSD area repeatability and 0.25% RSD
linearity. The Nexera Mikros system is ideal for low-level quantitation of peptides by LCMS.
retention time stability, extremely stable operation was demonstrated, without loss of peak shape common in high-throughput analyses of complex matrices.
Furthermore, the LCMS-8060NX with new ion guide UF-Qarray ™ II and the UF-Lens ™ II increases the robustness and minimizes downtime of the instrument.
Nanoparticle * Results obtained under our application conditions.
(FG beads Trypsin DART)
®
70,000
Trypsin-immobilized Diameter: LCMS Analysis
200 nm 60,000 Area Value: 4.38 %RSD
50,000
Collection of Fab-derived
Captured Antibody Fab Limited
Oriented toward Solution Trypsin Access peptide Area Value 40,000
Fab 30,000
20,000
• Decreases sample
Antibody complexity 10,000
• Prevents contamination
IgG collection from excess proteolysis 0 0 300 600 900 1,200 1,500
Number of Blood Plasma Injections
Diameter: 100 nm Area Repeatability of QC Samples
Immunoglobulin collection resin
5
The following is an example of antibody drug analysis in plasma. The plasma spiked with trastuzumab was pretreated using nSMOL Antibody BA Kit. 4
In the analysis of signature peptides deriving from trastuzumab, Nexera Mikros provides a dynamic range of 0.00763 – 62.5 µg/mL, good linearity with Retention Time: 0.25 %RSD
R > 0.99, and excellent accuracy of 101.0% (average). 3
2
100 2 800 Blank 800 0.00763 µg/mL Retention Time (min) 2
Calculated Conc. [µg/mL] * 1 0.1 1 R = 0.9998 600 600 1 0 0 300 Number of Blood Plasma Injections 1,200 1,500
10
600
900
400
400
0.01 200 200 Retention Time Reproducibility of QC Samples
0.01 0.1 1 10 100 0 0
Set Conc. [µg/mL] * 1 3.0 3.5 3.0 3.5 1,500th injection
Linearity (0.00763 to 62.5 µg/mL) Chromatogram (4 µL/min)
(×10,000) 750th injection
1.00
1st injection
The intra-day reproducibility of this trastuzumab-derived-peptide analysis is summarized in the table below. 0.75
These results show an excellent reproducibility, with both of accuracy and precision within 20% at LLOQ and within 15% even at other concentrations.
Concentration Setting QC set 1 * 2 QC set 2 * 2 0.50
(µg/mL) Accuracy Repeatability Accuracy Repeatability
0.00763 97.1% 5.69% 100% 11.3% 0.25
0.0229 102% 6.68% 101% 2.84%
5.86 106% 2.67% 99.4% 3.12% 0.00
50.0 94% 6.36% 91.7% 7.23% *1: This curve is drawn on logarithmic scale. 3.5 4.0 4.5 min
Intra-Day Reproducibility Evaluation Results from QC Samples *2: QC sets 1 and 2 were analyzed over a 2-day period,
with each set of concentrations analyzed five times. Chromatograms from 1st, 750th, and 1,500th Injections
Nexera Mikros
4 Microflow Liquid Chromatography Mass Spectrometry System 5