The Double-beam UV-Vis spectrophotometer offers excellent performance, data compliance, and intuitive operability
The UV-1900i Plus is a high-performance double-beam UV-Vis spectrophotometer with Shimadzu’s LO-RAY-LIGH™ diffraction grating technology. It offers 1 nm resolution, low stray light and noise, and ultra-fast scanning at 29,000 nm/min. The user-friendly multi-language interface supports various measurement modes, including photometric, spectrum, quantitation, kinetics, time-course, and bio-methods. It ensures high-accuracy quantitative analysis, detects low-concentration components, and complies with Pharmacopeia standards (JP, USP, EP).
With low stray light levels and high reproducibility, the UV-1900i Plus is ideal for accurate analysis of both low and high concentrations. It supports network connectivity for remote data checks (with additional memory) and can operate as a stand-alone unit with a color touch panel or via PC using LabSolutions UV-Vis software. Enhanced by a new CPU, it offers faster response speeds, efficient analysis, and integrated data management.

Refined User-Friendliness

Easy-to-Use Interface
Grasp the Current Status and Operating Procedures at a Glance
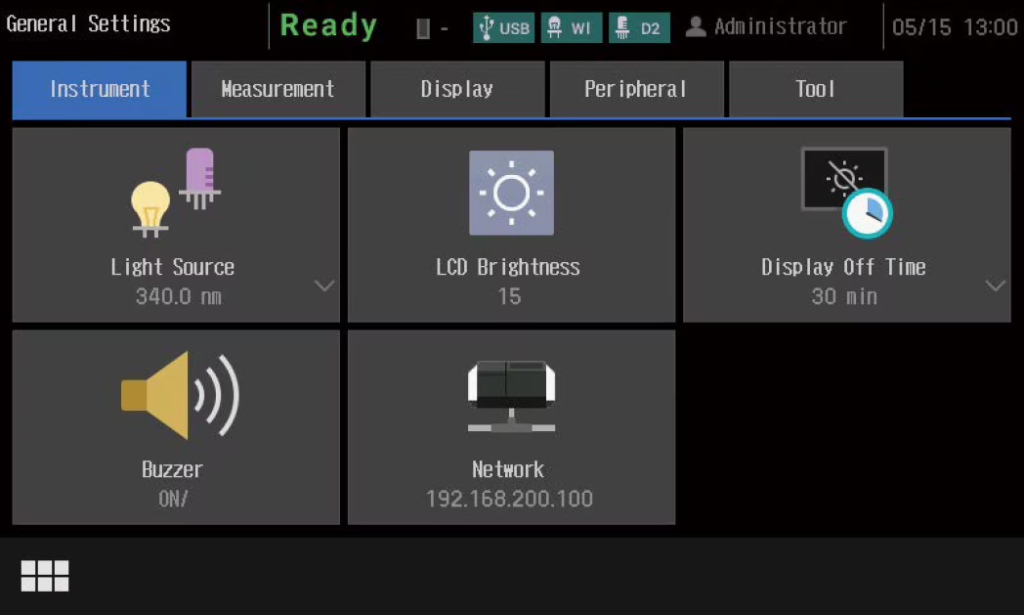
The UV-1900i Plus on-screen user interface includes large, easy-to-see icons deployed on a black background, so the instrument settings are evident at a glance. In addition, the large, easy-to-see icons improve intuitive understanding, which enables users to quickly become familiar with the operations. Furthermore, the user interface is designed to minimize transitions between windows, so users do not get confused during the operations. The system also features a faster response speed by adopting a new CPU.
Easy-to-Use Interface
Grasp the Current Status and Operating Procedures at a Glance
The UV-1900i Plus on-screen user interface includes large, easy-to-see icons deployed on a black background, so the instrument settings are evident at a glance. In addition, the large, easy-to-see icons improve intuitive understanding, which enables users to quickly become familiar with the operations. Furthermore, the user interface is designed to minimize transitions between windows, so users do not get confused during the operations. The system also features a faster response speed by adopting a new CPU.
Multiple Measurement Modes
UV-1900i Plus has six basic measurement modes, which offer the optimal solution for a variety of measurements.
Photometric
Measures the photometric value at a single wavelength or multiple (up to eight) wavelengths.
Spectrum
Measures a sample spectrum using wavelength scanning.

Quantitation
Generates a calibration curve from the measurement of standards, and then calculates the concentrations of unknowns.

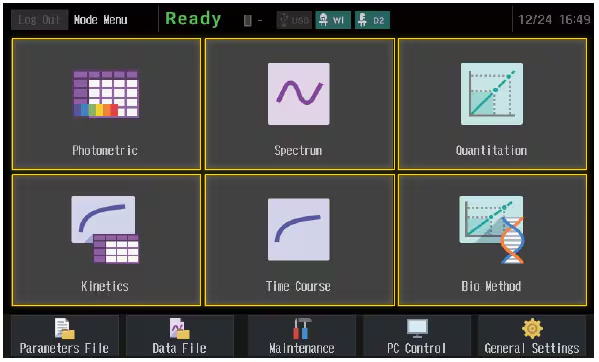

Kinetics
Measures absorbance changes as a function of time and obtains the enzymatic activity values. The kinetics measurement method or the rate measurement method can be selected
 Time CourseMeasures changes over time in photometric values at a specified wavelength.
Time CourseMeasures changes over time in photometric values at a specified wavelength.
 BiomethodQuantifies DNA or protein concentrations.
BiomethodQuantifies DNA or protein concentrations.
* The background color of the operation screen can also be set to white.
Various Functions for Comfortable Daily Measurement
- Assist Function The instrument assists the user to ensure that measurements
are performed using the correct procedure.*1
 Checks whether or not baseline correction, auto zero correction or cell blank correction has been performed and informs the user if it has not been.
Checks whether or not baseline correction, auto zero correction or cell blank correction has been performed and informs the user if it has not been.

Informs the user if the last correction performed is not appropriate for the planned measurement
 Informs the user if the UV-1900i Plus is not ready to begin measurement and when starting measurements
Informs the user if the UV-1900i Plus is not ready to begin measurement and when starting measurements
and 100 %T (0 Abs) corrections.
*1 This function can be enabled/disabled.
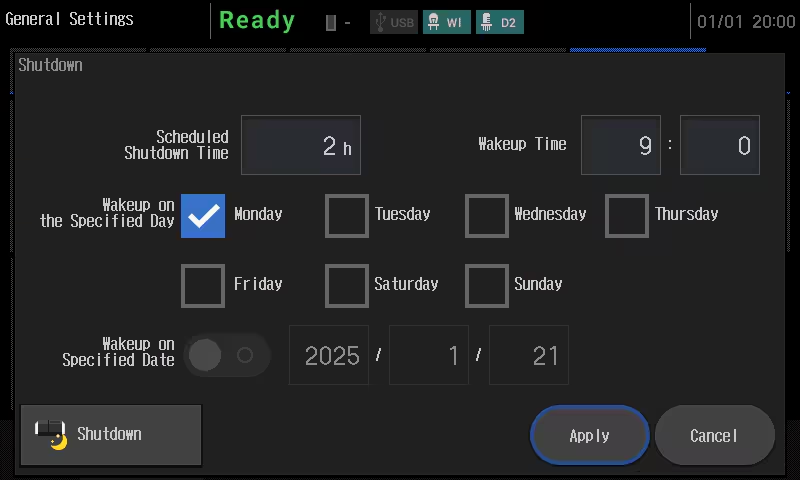
Shutdown Function
The shutdown function puts UV-1900i Plus to sleep after it’s been used, or after a certain period of time. Putting the instrument into sleep mode limits power consumption and helps preserve the lamp.

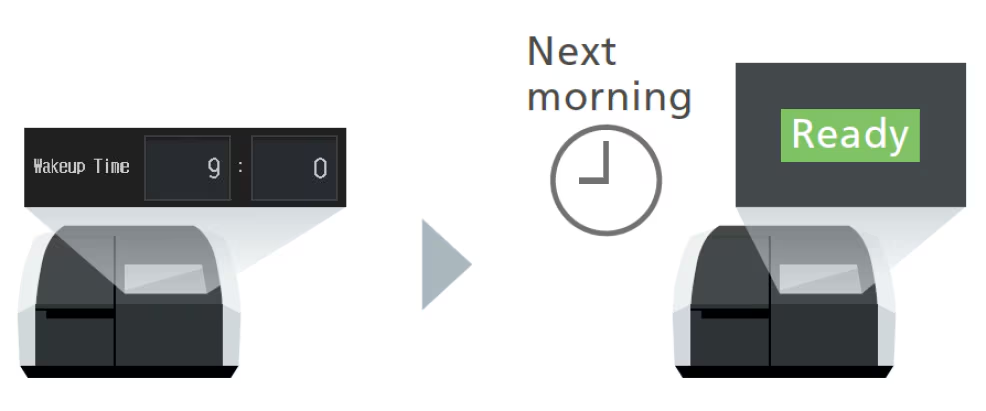
Wakeup Function
Automatically wakes up a sleeping UV-1900i Plus at a specified time. This function eliminates the need for analysts to wait for it to warm up, allowing them to be more productive.
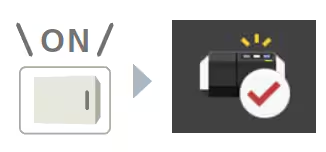
Startup Validation Function
Instrument performance checks can be performed automatically when the instrument is turned ON.*2
This enables even more reliable instrument operation.
It can also be implemented in combination with the Wakeup function.
*2 This function can be enabled/disabled.
The performance check items that can be used with this function are as follows: wavelength accuracy (D2), wavelength repeat accuracy (D2), degradation, noise level, baseline flatness, baseline stability (drift), [EP] Control of Wavelength Accuracy (D2), and [USP] Control of Wavelengths (D2).
Network Connectivity Function
Data can now be transferred to a PC via a network. With wireless printing, multiple UV units can print from a single printer.*3 (A router and other network equipment must be installed to use a network.)
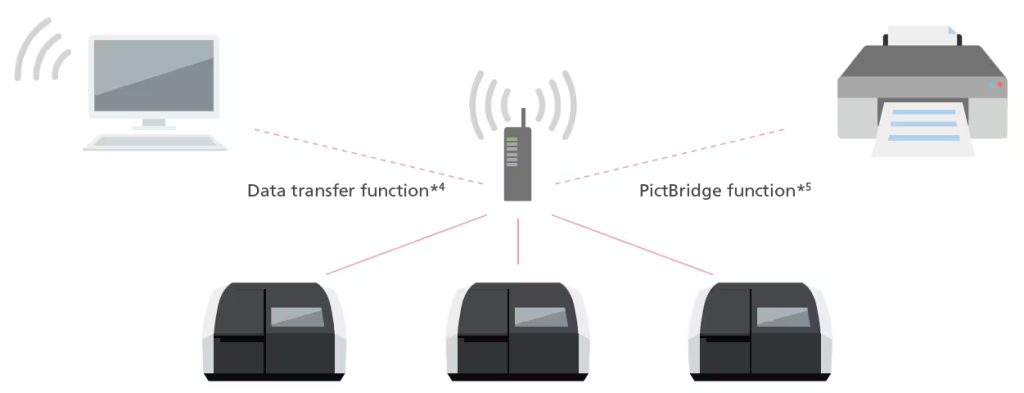
*3 A router with a wireless function is required.
*4 Optional expanded memory is required. The instrument is not compatible with control via a network.
*5 A PictBridge-compatible printer is required.
Bar-Code Reader and Keyboard Entry Function
Sample names and numerical values can be entered by a bar-code reader or from the keyboard.
This saves time when entering sample names for a multiple-sample analysis, and prevents sample misidentification and other human errors.*6

*6 Use a bar-code reader and keyboard with a USB connection.
High Performance to Meet Diverse Needs
Ultra-Fast Scan
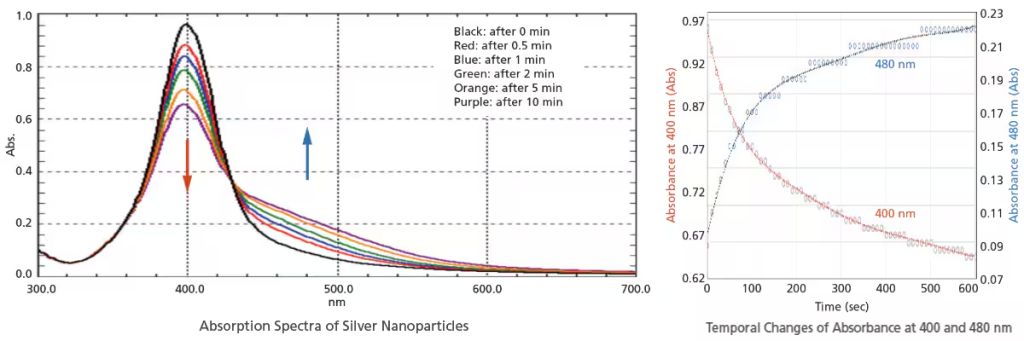
Spectra can be acquired as fast as 29,000 nm/min. Ultra-fast scanning is effective in tracking chemical reactions in a short time. In addition to the absorbance change at specified wavelengths, spectra can also be acquired in a short time with the UV-1900i Plus. Therefore, more detailed behavior can be investigated by observing spectra with the UV-1900i Plus.
The figures below show the analysis of the particle agglomeration process when salts are added to silver nanoparticles. Measurements of the 300 to 700 nm region were performed in ultra-fast scan mode. In addition to the decrease of absorbance at 400 nm and the increase of absorbance at 480 nm, the temporal changes of spectra can also be observed.
Low Stray Light
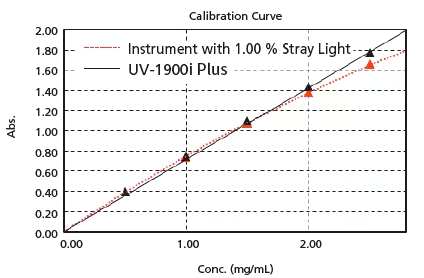
Linearity will be lost in the high absorbance region due to stray light. UV-1900i Plus is equipped with Low Stray Light Diffraction Gratings, achieve “best-in-class” low stray light. Stray light is at 0.5 % max. (198 nm), making accurate measurements possible at 1 Abs or more, even in the ultraviolet region. In addition, high-concentration samples can be quantified accurately.
The figure on the right is a calibration curve for acetic acid, created with absorbance at 200 nm. The correlation coefficient is 0.9998 and correct measured values are obtained even with high-concentration samples.
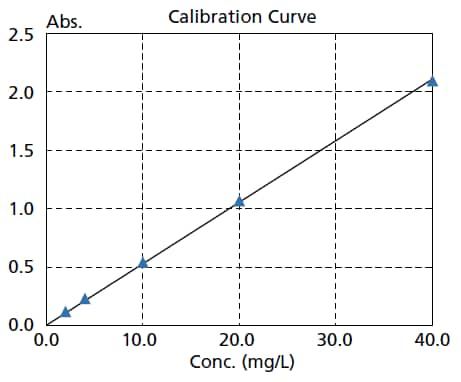
High Reproducibility and Repeatability Accuracy
The photometric repeatability accuracy is 0.0002 Abs max. (0.5 Abs and 1.0 Abs). As a result, variance in the measurement results is suppressed, enabling more accurate quantitation and the detection of low-concentration samples.
The figure on the right is a calibration curve for caffeine, created with absorbance at 273 nm.
The calibration curve has an Abs = 0.0528 Conc. The lower limit of quantitation determined from the standard deviation is 0.0051 mg/L.
Note: One method of determining the lower limit of quantitation is to use ten times the standard deviation. This is an actual measured value and is not guaranteed.
| No. | Absorbance of Blank Solution(273 nm) |
| 1 | -0.00001 |
| 2 | 0.00001 |
| 3 | -0.00002 |
| 4 | 0.00002 |
| 5 | 0.00001 |
| 6 | -0.00003 |
| 7 | 0.00001 |
| 8 | -0.00004 |
| 9 | 0.00001 |
| 10 | 0.00005 |
| Standard Deviationσ | 0.000025 |
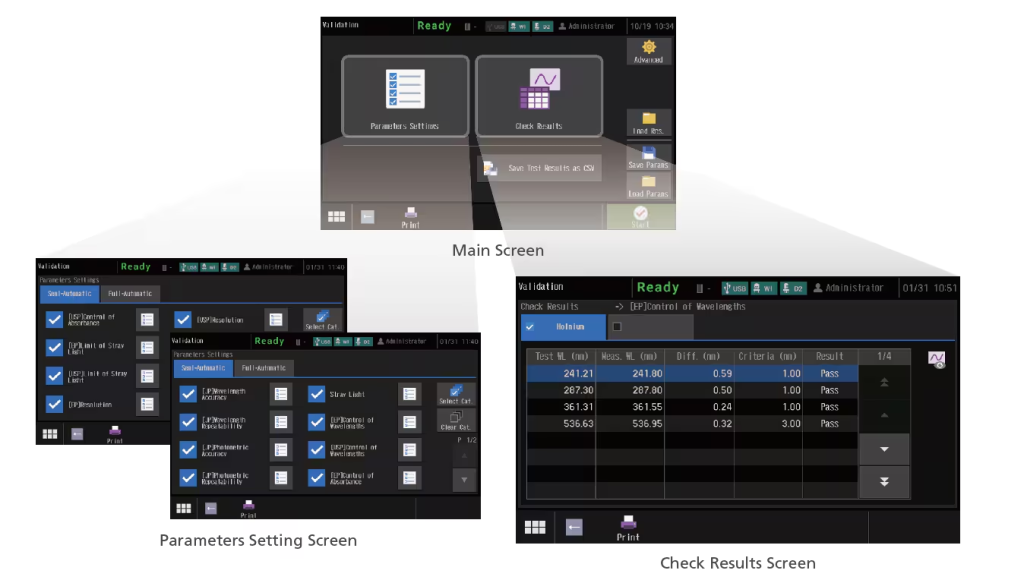
Validation Functions Compliant with JP, USP, and EP (Touch-screen Display)
This instrument can not only run checks for nine JIS items, but also those stipulated in the Japanese Pharmacopoeia (JP), United States Pharmacopeia (USP), and the European Pharmacopoeia (EP). The UV-1900 series also complies with the hardware specifications required by each Pharmacopoeia. In addition, the check conditions can be saved. As a result, checks can be performed easily just by calling them up as needed. Check results can also be saved.
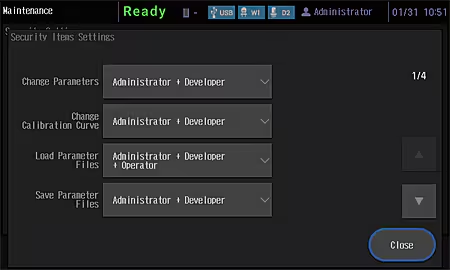
Improved Security Functions
An external control security function has been added to provide more support for compliance with regulations. Three user authority levels, “Administrator”, “Developer”, and “Operator”, can be set for instrument users.
Resolution of 1 nm, the Highest in its Class
In addition to achieving a resolution of 1 nm, the highest in its class, by using a monochromator with a Czerny-Turner mounting, the UV-1900i Plus also features a compact, bright optical system. The instrument is more than capable of meeting the wavelength resolution required in the USP and EP.
Compatible with Validation from PC Software
UV validation software (limited function edition) is included as standard. Using this software, inspections can be implemented as stipulated for 9 items in JIS and the JP. If optional UV validation software (USP/EP compatible edition) is added, examinations can be implemented as stipulated in the USP and the EP.
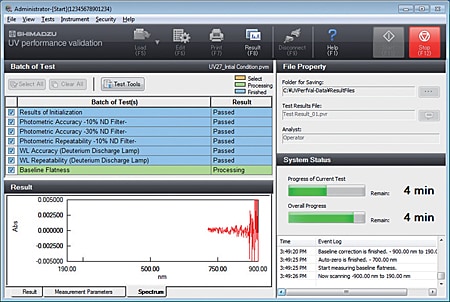
- Inspection results can not only be printed but also saved to a file, so the results can be called up later for confirmation.
- The inspection parameters can also be saved to separate files for periodic and routine inspections, and then called up for use.
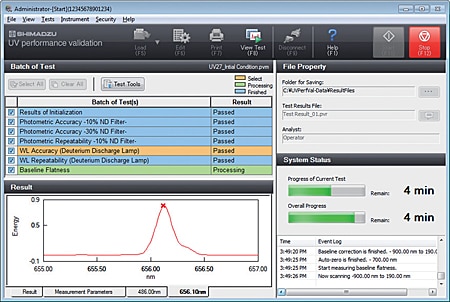
- The user can select confirmation of instrument performance indicators as per JIS K0115 General rules for molecular absorptiometric analysis, as well as the general test methods in the JP. (Order inspection jigs and reagents separately.)
Note: Optional validation software is required to support the USP and EP.
- The user can select confirmation of instrument performance indicators as per JIS K0115 General rules for molecular absorptiometric analysis, as well as the general test methods in the JP. (Order inspection jigs and reagents separately.)
Support for FDA 21 CFR Part 11, PIC/S GMP Guidelines and Other Regulations and Guidelines
Ensuring the integrity of data (database management), including the user management, user authority management, and data audit trails required for compliance with FDA 21 CFR Part 11, PIC/S GMP guidelines, and other ER/ES regulations is possible.
Reliable LabSolutions Software
In addition to LabSolutions UV-Vis, which provides basic functionality, Shimadzu also offers LabSolutions DB UV-Vis and LabSolutions CS UV-Vis to meet the requirements of ER/ES regulations.